Abstract
Carboxypeptidase A1 (CPA1) is a zinc metalloprotease that is produced in pancreatic acinar cells and plays a role in cleaving C-terminal branched-chain and aromatic amino acids from dietary proteins. This study assessed the utility of immunohistochemical CPA1 staining for diagnosing pancreatic acinar cell carcinoma (ACC). A total of 12,274 tumor samples from 132 different tumor types and subtypes as well as 8 samples each of 76 different normal tissue types were interpretable by immunohistochemistry in a tissue microarray format. CPA1 was strongly expressed in acinar cells of all normal pancreas samples but not in any other normal tissues. CPA1 immunostaining was detected in 100% of 11 pancreatic ACCs and 1 mixed acinar endocrine carcinoma, but absent in 449 pancreatic ductal adenocarcinomas, 75 adenocarcinomas of the ampulla Vateri, and 11,739 other evaluable cancers from 128 different tumor entities. A weak to moderate diffuse staining of epithelial and stromal cells of cancer tissues immediately adjacent to non-neoplastic pancreatic acinar cells often occurred and was considered to be caused by the diffusion of the highly abundant CPA1 from normal acinar cells that may have suffered some autolytic cell damage. In conclusion, our data show that CPA1 is a highly sensitive and largely specific marker for normal and neoplastic pancreatic acinar cells. CPA1 immunohistochemistry greatly facilitates the otherwise often difficult diagnosis of pancreatic ACC.
Key Words: carboxypeptidase A1, immunohistochemistry, tissue micro array, acinar cell carcinoma, pancreas
Acinar cell carcinoma (ACC) of the pancreas represents a rare cancer type derived from pancreatic acinar cells that accounts for <2% of all pancreatic neoplasms.1 Most ACCs occur as pure ACCs, but mixed carcinomas with >25% of additional cell types also occur and include mixed acinar-neuroendocrine carcinoma (MAEC) and mixed acinar-ductal carcinoma.2 A recent analysis of 57,804 pancreatic cancer patients who underwent surgical resection showed a median overall survival (mOS) of 67.5 months and 51% 5-year OS for ACC.3 In case reports, OS reached up to 123 months.4 Accordingly, the prognosis of pancreatic ACC is markedly better than the 22% 5-year OS of resected ductal adenocarcinoma but significantly worse than the 84% 5-year OS of neuroendocrine tumors (NETs) of the pancreas.3 Considerable differences in the molecular alterations seen between these tumor entities are likely to be responsible for marked differences in cancer aggressiveness and also in response to chemotherapy.5 A precise distinction of these pancreatic tumor entities by the pathologist is thus of high importance.
Due to its relatively broad morphologic spectrum caused by some morphologic and immunohistochemical overlap with NETs, ACC can represent a difficult diagnosis for pathologists, and false diagnoses do occur both on histologic and cytologic samples.6–8 The correct diagnosis in cases with nonprototypic morphology requires the use of immunohistochemical markers such as trypsin, chymotrypsin, BCL-10, cytokeratin 19, and cytokeratin 7.9 However, none of these antibodies are completely sensitive and specific for ACC.10,11 Carboxypeptidase A1 (CPA1) is a zinc metalloprotease coded by the CPA1 gene located at 7q32.2. It is a 34.6 kDa protein which is produced in the pancreatic acinar cells. It is involved in zymogen inhibition and was shown to preferentially cleave C-terminal branched-chain and aromatic amino acids from dietary proteins.12 Mutations of CPA1 gene have been linked to chronic pancreatitis.13,14 Elevated CPA1 serum protein levels have been described in patients with pancreatic cancer.15 CPA1 immunohistochemistry may be suited for facilitating a safe diagnosis of ACC. In a recent study, analyzing 14 ACC and 5 MAEC as well as 80 nonacinar pancreatic tumors, Said et al16 found a 100% sensitivity and a 95% specificity of a positive CPA1 immunostaining for ACC.
To further assess the potential of CPA1 immunostaining for securing the diagnosis of pancreatic ACC an extensive survey of CPA1 immunostaining in nonpancreatic tumor types is needed. In this study, CPA1 expression was thus analyzed in 15,680 tumor tissue samples from 132 different tumor types and subtypes as well as 76 different non-neoplastic tissue types by immunohistochemistry in a tissue microarray (TMA) format.
MATERIALS AND METHODS
Tissue Microarrays
Preexisting TMAs, which have been used in several previous studies17–20 were also used for this study. Our normal tissue TMA was composed of 8 samples from 8 different donors for each of 76 different normal tissue types (608 samples on 1 slide). The cancer TMAs contained a total of 15,680 primary tumors from 132 tumor types and subtypes. The composition of both normal and cancer TMAs is described in detail in the Results section. Our “pancreatic heterogeneity TMA” contained up to 9 samples (mean: 6.7) each from 224 pancreatic ductal adenocarcinomas that were taken from 3 to 9 (mean: 5.5) available tumor containing tissue blocks per patient. These tumors partially overlapped with the set analyzed in the primary tumor TMAs. All samples were from the archives of the Institutes of Pathology, University Hospital of Hamburg, Germany, the Institute of Pathology, Clinical Center Osnabrueck, Germany, and Department of Pathology, Academic Hospital Fuerth, Germany. Tissues were fixed in 4% buffered formalin and then embedded in paraffin. Decalcification was not performed on any tissue samples. Time of tissue acquisition to fixation and fixation duration was not standardized. TMA tissue spot diameter was 0.6 mm. The use of archived remnants of diagnostic tissues for manufacturing of TMAs and their analysis for research purposes as well as patient data analysis has been approved by local laws (HmbKHG, §12) and by the local ethics committee (Ethics commission Hamburg, WF-049/09). All work has been carried out in compliance with the Helsinki Declaration.
Immunohistochemistry
Freshly cut TMA sections were immunostained on 1 day and in 1 experiment. Slides were deparaffinized with xylol, rehydrated through a graded alcohol series, and exposed to heat-induced antigen retrieval for 5 minutes in an autoclave at 121°C in pH 7.8 DakoTarget Retrieval Solution (Agilent, California; #S2367). Endogenous peroxidase activity was blocked with Dako Peroxidase Blocking Solution (Agilent; #52023) for 10 minutes. A primary antibody specific for CPA1 (mouse monoclonal, MSVA-601M, MS Validated Antibodies, Hamburg, Germany, ms-validatedantibodies.com) was applied at 37°C for 60 minutes at a dilution of 1:150. Bound antibody was then visualized using the EnVision Kit (Agilent; #K5007) according to the manufacturer’s directions. The sections were counterstained with haemalaun. One pathologist (S.M.) evaluated all TMA slides. For normal tissues, the immunostaining staining intensity of positive cells was semi-quantitively recorded (+, ++, +++). For tumor tissues, the percentage of positive neoplastic cells was estimated, and the immunostaining staining intensity was semiquantitatively recorded (0, 1+, 2+, 3+). For statistical analyses, the staining results were categorized into 4 groups. Tumors without any staining were considered negative. Tumors with 1+ staining intensity in ≤70% of cells and 2+ intensity in ≤30% of cells were considered weakly positive. Tumors with 1+ staining intensity in >70% of cells, 2+ intensity in 31% to 70%, or 3+ intensity in ≤30% were considered moderately positive. Tumors with 2+ intensity in >70% or 3+ intensity in >30% of cells were considered strongly positive.
RESULTS
Technical Issues
A total of 12,274 (78.3%) of 15,680 tumor samples were interpretable in our TMA analysis. Noninterpretable samples demonstrated a lack of unequivocal tumor cells or loss of the tissue spots during technical procedures. At least 4 samples of each normal tissue type were evaluable.
CPA1 in Normal Tissues
A strong (3+) cytoplasmatic CPA1 staining was only found in the pancreas where staining was most intense in acinar cells (Fig. 1). However, in cases with particularly strong acinar cell positivity, some fading of the staining into adjacent other cell types was occasionally observed. CPA1 staining was completely absent in all analyzed extrapancreatic tissues including skeletal muscle, heart muscle, smooth muscle, myometrium of the uterus, corpus spongiosum of the penis, ovarian stroma, fat, skin (including hair follicle and sebaceous glands), oral mucosa of the lip, oral cavity, surface epithelium of the tonsil, and transitional mucosa of the anal canal, ectocervix, squamous epithelium of the esophagus, urothelium of the renal pelvis and urinary bladder, decidua, placental trophoblastic cells, lymph node, spleen, thymus, tonsil, mucosa of the stomach, duodenum, ileum, appendix, colon, rectum and gall bladder, liver, parotid gland, submandibular gland, sublingual gland, Brunner gland of the duodenum, cortex and medulla of the kidney, prostate, seminal vesicle, epididymis, testis, respiratory epithelium and glands of bronchi and sinus paranasales, lung, breast, endocervix, endometrium, fallopian tube, corpus luteum and follicular cyst of the ovary, adrenal gland, parathyroid gland, cerebellum, cerebrum, and pituitary gland.
FIGURE 1.
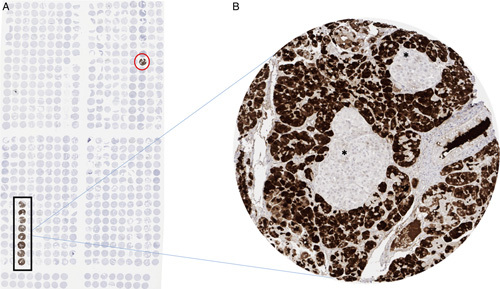
CPA1 immunostaining in normal tissues. A, Overview of the normal tissue microarray. Eight spots of acinar parenchyma in 1 row show a strong CPA1-staining (black rectangular). In the row of thymic tissue, acinar parenchyma has been mistakenly used (red circle). None of the other normal tissues show any positive CPA1-staining. B, Enlargement of one tissue microarray spot showing strong CPA1-staining in acinar parenchyma of the pancreas. Absent staining in Islets of Langerhans*.
CPA1 in Neoplastic Tissues
A strong cytoplasmic CPA1 immunostaining was observed in 6 of 6 previously diagnosed ACC (Fig. 2). A strong CPA1 immunostaining was also seen in 5 tumors initially classified as NET (G3; n=1), neuroendocrine carcinoma (NEC; n=2) or adenocarcinoma of the pancreas (n=2), and in 1 tumor initially classified as NEC, large cell variant. However, after comprehensive histologic reevaluation by 4 pathologists (S.M., G.S., T.S.C., and F.J.), these tumors were subsequently reclassified as pancreatic ACC and mixed acinar endocrine carcinoma (MAEC) (see the Discussion section). CPA1 immunostaining was completely absent in all other 12,262 evaluable tumors from 130 different tumor types and subtypes (Table 1). This included 128 evaluable acinic cell carcinomas derived from salivary glands.
FIGURE 2.

CPA1 immunostaining in tumors. A, Strong positive CPA1 staining in acinar cell carcinoma. B, Absent CPA1 staining in a neuroendocrine tumor of the pancreas. Positive staining in non-neoplastic acinar parenchyma of the pancreas.
TABLE 1.
CPA1 Immunostaining in Tumors
| CPA1 Immunostaining | |||||||
|---|---|---|---|---|---|---|---|
| Tumor Entity | On TMA (n) | Analyzable (n) | Negative (%) | Weak (%) | Moderate (%) | Strong (%) | |
| Tumors of the skin | Pilomatrixoma | 35 | 32 | 100 | 0 | 0 | 0 |
| Basal cell carcinoma | 88 | 56 | 100 | 0 | 0 | 0 | |
| Benign nevus | 29 | 26 | 100 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the skin | 90 | 82 | 100 | 0 | 0 | 0 | |
| Malignant melanoma | 48 | 44 | 100 | 0 | 0 | 0 | |
| Merkel cell carcinoma | 46 | 44 | 100 | 0 | 0 | 0 | |
| Tumors of the head and neck | Squamous cell carcinoma of the larynx | 110 | 96 | 100 | 0 | 0 | 0 |
| Squamous cell carcinoma of the pharynx | 60 | 46 | 100 | 0 | 0 | 0 | |
| Oral squamous cell carcinoma (floor of the mouth) | 130 | 118 | 100 | 0 | 0 | 0 | |
| Pleomorphic adenoma of the parotid gland | 50 | 44 | 100 | 0 | 0 | 0 | |
| Warthin tumor of the parotid gland | 104 | 91 | 100 | 0 | 0 | 0 | |
| Adenocarcinoma, not otherwise specified (NOS) (papillary cystadenocarcinoma) | 14 | 12 | 100 | 0 | 0 | 0 | |
| Salivary duct carcinoma | 15 | 12 | 100 | 0 | 0 | 0 | |
| Acinic cell carcinoma of the salivary gland | 181 | 128 | 100 | 0 | 0 | 0 | |
| Adenocarcinoma NOS of the salivary gland | 109 | 69 | 100 | 0 | 0 | 0 | |
| Adenoid cystic carcinoma of the salivary gland | 180 | 99 | 100 | 0 | 0 | 0 | |
| Basal cell adenocarcinoma of the salivary gland | 25 | 21 | 100 | 0 | 0 | 0 | |
| Basal cell adenoma of the salivary gland | 101 | 86 | 100 | 0 | 0 | 0 | |
| Epithelial-myoepithelial carcinoma of the salivary gland | 53 | 51 | 100 | 0 | 0 | 0 | |
| Mucoepidermoid carcinoma of the salivary gland | 343 | 239 | 100 | 0 | 0 | 0 | |
| Myoepithelial carcinoma of the salivary gland | 21 | 20 | 100 | 0 | 0 | 0 | |
| Myoepithelioma of the salivary gland | 11 | 9 | 100 | 0 | 0 | 0 | |
| Oncocytic carcinoma of the salivary gland | 12 | 12 | 100 | 0 | 0 | 0 | |
| Polymorphous adenocarcinoma, low grade, of the salivary gland | 41 | 32 | 100 | 0 | 0 | 0 | |
| Pleomorphic adenoma of the salivary gland | 53 | 32 | 100 | 0 | 0 | 0 | |
| Tumors of the lung, pleura, and thymus | Adenocarcinoma of the lung | 246 | 160 | 100 | 0 | 0 | 0 |
| Squamous cell carcinoma of the lung | 130 | 65 | 100 | 0 | 0 | 0 | |
| Small cell carcinoma of the lung | 20 | 16 | 100 | 0 | 0 | 0 | |
| Mesothelioma, epitheloid | 39 | 32 | 100 | 0 | 0 | 0 | |
| Mesothelioma, other types | 76 | 63 | 100 | 0 | 0 | 0 | |
| Thymoma | 29 | 29 | 100 | 0 | 0 | 0 | |
| Tumors of the female genital tract | Squamous cell carcinoma of the vagina | 78 | 63 | 100 | 0 | 0 | 0 |
| Squamous cell carcinoma of the vulva | 130 | 116 | 100 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the cervix | 130 | 124 | 100 | 0 | 0 | 0 | |
| Endometrioid endometrial carcinoma | 236 | 223 | 100 | 0 | 0 | 0 | |
| Endometrial serous carcinoma | 82 | 72 | 100 | 0 | 0 | 0 | |
| Carcinosarcoma of the uterus | 48 | 38 | 100 | 0 | 0 | 0 | |
| Endometrial carcinoma, high grade, G3 | 13 | 13 | 100 | 0 | 0 | 0 | |
| Endometrial clear cell carcinoma | 8 | 7 | 100 | 0 | 0 | 0 | |
| Endometrioid carcinoma of the ovary | 110 | 91 | 100 | 0 | 0 | 0 | |
| Serous carcinoma of the ovary | 559 | 462 | 100 | 0 | 0 | 0 | |
| Mucinous carcinoma of the ovary | 96 | 71 | 100 | 0 | 0 | 0 | |
| Clear cell carcinoma of the ovary | 50 | 40 | 100 | 0 | 0 | 0 | |
| Carcinosarcoma of the ovary | 47 | 38 | 100 | 0 | 0 | 0 | |
| Brenner tumor | 9 | 9 | 100 | 0 | 0 | 0 | |
| Tumors of the breast | Invasive breast carcinoma of no special type | 1391 | 1185 | 100 | 0 | 0 | 0 |
| Lobular carcinoma of the breast | 294 | 236 | 100 | 0 | 0 | 0 | |
| Medullary carcinoma of the breast | 26 | 26 | 100 | 0 | 0 | 0 | |
| Tubular carcinoma of the breast | 27 | 26 | 100 | 0 | 0 | 0 | |
| Mucinous carcinoma of the breast | 58 | 44 | 100 | 0 | 0 | 0 | |
| Phyllodes tumor of the breast | 50 | 47 | 100 | 0 | 0 | 0 | |
| Tumors of the digestive system | Adenomatous polyp, low-grade dysplasia | 50 | 49 | 100 | 0 | 0 | 0 |
| Adenomatous polyp, high-grade dysplasia | 50 | 49 | 100 | 0 | 0 | 0 | |
| Adenocarcinoma of the colon | 956 | 721 | 100 | 0 | 0 | 0 | |
| Gastric adenocarcinoma, diffuse type | 226 | 129 | 100 | 0 | 0 | 0 | |
| Gastric adenocarcinoma, intestinal type | 224 | 134 | 100 | 0 | 0 | 0 | |
| Gastric adenocarcinoma, mixed type | 62 | 47 | 100 | 0 | 0 | 0 | |
| Adenocarcinoma of the esophagus | 133 | 60 | 100 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the esophagus | 124 | 42 | 100 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the anal canal | 91 | 78 | 100 | 0 | 0 | 0 | |
| Cholangiocarcinoma | 114 | 108 | 100 | 0 | 0 | 0 | |
| Hepatocellular carcinoma | 50 | 50 | 100 | 0 | 0 | 0 | |
| Ductal adenocarcinoma of the pancreas | 662 | 449 | 100 | 0 | 0 | 0 | |
| Pancreatic/Ampullary adenocarcinoma | 119 | 75 | 100 | 0 | 0 | 0 | |
| Acinar cell carcinoma of the pancreas | 11 | 11 | 0 | 0 | 18.2 | 81.8 | |
| Mixed acinar endocrine carcinoma of the pancreas | 1 | 1 | 0 | 0 | 0 | 100 | |
| Gastrointestinal stromal tumor (GIST) | 50 | 49 | 100 | 0 | 0 | 0 | |
| Tumors of the urinary system | Urothelial carcinoma, pT2-4 G3 | 1207 | 613 | 100 | 0 | 0 | 0 |
| Small cell neuroendocrine carcinoma of the bladder | 18 | 18 | 100 | 0 | 0 | 0 | |
| Sarcomatoid urothelial carcinoma | 25 | 24 | 100 | 0 | 0 | 0 | |
| Clear cell renal cell carcinoma | 858 | 759 | 100 | 0 | 0 | 0 | |
| Papillary renal cell carcinoma | 255 | 208 | 100 | 0 | 0 | 0 | |
| Clear cell (tubulo) papillary renal cell carcinoma | 21 | 19 | 100 | 0 | 0 | 0 | |
| Chromophobe renal cell carcinoma | 131 | 118 | 100 | 0 | 0 | 0 | |
| Oncocytoma | 177 | 147 | 100 | 0 | 0 | 0 | |
| Tumors of the male genital organs | Adenocarcinoma of the prostate, Gleason 3+3 | 83 | 79 | 100 | 0 | 0 | 0 |
| Adenocarcinoma of the prostate, Gleason 4+4 | 80 | 73 | 100 | 0 | 0 | 0 | |
| Adenocarcinoma of the prostate, Gleason 5+5 | 85 | 80 | 100 | 0 | 0 | 0 | |
| Adenocarcinoma of the prostate (recurrence) | 261 | 231 | 100 | 0 | 0 | 0 | |
| Small cell neuroendocrine carcinoma of the prostate | 17 | 16 | 100 | 0 | 0 | 0 | |
| Seminoma | 621 | 444 | 100 | 0 | 0 | 0 | |
| Embryonal carcinoma of the testis | 50 | 39 | 100 | 0 | 0 | 0 | |
| Yolk sack tumor | 50 | 32 | 100 | 0 | 0 | 0 | |
| Teratoma | 50 | 44 | 100 | 0 | 0 | 0 | |
| Squamous cell carcinoma of the penis | 80 | 66 | 100 | 0 | 0 | 0 | |
| Tumors of endocrine organs | Adenoma of the thyroid gland | 114 | 108 | 100 | 0 | 0 | 0 |
| Papillary thyroid carcinoma | 392 | 361 | 100 | 0 | 0 | 0 | |
| Follicular thyroid carcinoma | 158 | 147 | 100 | 0 | 0 | 0 | |
| Medullary thyroid carcinoma | 107 | 100 | 100 | 0 | 0 | 0 | |
| Anaplastic thyroid carcinoma | 45 | 43 | 100 | 0 | 0 | 0 | |
| Adrenal cortical adenoma | 50 | 44 | 100 | 0 | 0 | 0 | |
| Adrenal cortical carcinoma | 26 | 26 | 100 | 0 | 0 | 0 | |
| Phaeochromocytoma | 50 | 50 | 100 | 0 | 0 | 0 | |
| Appendix, neuroendocrine tumor (NET) | 22 | 12 | 100 | 0 | 0 | 0 | |
| Colorectal, NET | 11 | 10 | 100 | 0 | 0 | 0 | |
| Ileum, NET | 49 | 46 | 100 | 0 | 0 | 0 | |
| Lung, NET | 19 | 17 | 100 | 0 | 0 | 0 | |
| Pancreas, NET | 98 | 87 | 100 | 0 | 0 | 0 | |
| Colorectal, NEC | 12 | 10 | 100 | 0 | 0 | 0 | |
| Gallbladder, NEC | 4 | 4 | 100 | 0 | 0 | 0 | |
| Pancreas, NEC | 14 | 14 | 100 | 0 | 0 | 0 | |
| Tumors of hematopoietic and lymphoid tissues | Hodgkin lymphoma | 103 | 76 | 100 | 0 | 0 | 0 |
| Non-Hodgkin lymphoma | 62 | 54 | 100 | 0 | 0 | 0 | |
| Small lymphocytic lymphoma, B-cell type (B-SLL/B-CLL) | 50 | 30 | 100 | 0 | 0 | 0 | |
| Diffuse large B-cell lymphoma (DLBCL) | 114 | 94 | 100 | 0 | 0 | 0 | |
| Follicular lymphoma | 88 | 63 | 100 | 0 | 0 | 0 | |
| T-cell non-Hodgkin lymphoma | 24 | 16 | 100 | 0 | 0 | 0 | |
| Mantle cell lymphoma | 18 | 13 | 100 | 0 | 0 | 0 | |
| Marginal zone lymphoma | 16 | 10 | 100 | 0 | 0 | 0 | |
| DLBCL in the testis | 16 | 13 | 100 | 0 | 0 | 0 | |
| Burkitt lymphoma | 5 | 1 | 100 | 0 | 0 | 0 | |
| Tumors of soft tissue and bone | Tenosynovial giant cell tumor | 45 | 44 | 100 | 0 | 0 | 0 |
| Granular cell tumor | 53 | 44 | 100 | 0 | 0 | 0 | |
| Leiomyoma | 50 | 48 | 100 | 0 | 0 | 0 | |
| Leiomyosarcoma | 87 | 84 | 100 | 0 | 0 | 0 | |
| Liposarcoma | 132 | 129 | 100 | 0 | 0 | 0 | |
| Malignant peripheral nerve sheath tumor (MPNST) | 13 | 11 | 100 | 0 | 0 | 0 | |
| Myofibrosarcoma | 26 | 26 | 100 | 0 | 0 | 0 | |
| Angiosarcoma | 73 | 66 | 100 | 0 | 0 | 0 | |
| Angiomyolipoma | 91 | 91 | 100 | 0 | 0 | 0 | |
| Dermatofibrosarcoma protuberans | 21 | 18 | 100 | 0 | 0 | 0 | |
| Ganglioneuroma | 14 | 13 | 100 | 0 | 0 | 0 | |
| Kaposi sarcoma | 8 | 6 | 100 | 0 | 0 | 0 | |
| Neurofibroma | 117 | 96 | 100 | 0 | 0 | 0 | |
| Sarcoma, NOS | 75 | 59 | 100 | 0 | 0 | 0 | |
| Paraganglioma | 41 | 37 | 100 | 0 | 0 | 0 | |
| Primitive neuroectodermal tumor (PNET) | 23 | 18 | 100 | 0 | 0 | 0 | |
| Rhabdomyosarcoma | 7 | 7 | 100 | 0 | 0 | 0 | |
| Schwannoma | 121 | 106 | 100 | 0 | 0 | 0 | |
| Synovial sarcoma | 12 | 11 | 100 | 0 | 0 | 0 | |
| Osteosarcoma | 43 | 35 | 100 | 0 | 0 | 0 | |
| Chondrosarcoma | 38 | 17 | 100 | 0 | 0 | 0 | |
All reclassified cases are included in this table. All positive cases are highlighted in gray.
CPA1 Heterogeneity Analyses
In an attempt to identify focal CPA1 positivity in pancreatic tumors, 17 large sections of ductal adenocarcinomas and 20 large section of NETs were analyzed as well as a heterogeneity TMA containing up to 9 samples from 224 pancreatic ductal adenocarcinomas. In 219 of 224 primary tumors a minimum of 2 of 6 samples were evaluable (a minimum of 3 spots were evaluable in 206, ≥4 in 182, ≥5 in 134 and all 6 in 56 primary tumors). The range of evaluable samples was 0 to 6 for primary tumors and 0 to 3 for metastasis. A strong CPA1 immunostaining was found in 1 ACC (case #5, see above). In the remaining 223 tumors CPA1 staining was not observed in any tumor cells of these samples. In several instances, however, a weak to moderate CPA1 staining was seen immediately adjacent to highly CPA1 expressing normal pancreatic tissue that equally involved tumor and stromal cells. This staining was considered to reflect diffusion of highly abundant CPA1 from normal acinar cells that may have suffered some autolytic cell damage.
DISCUSSION
The results of this study demonstrate a close to perfect sensitivity and specificity of anti-CPA1 clone MSVA-601M for diagnosing pancreatic ACC.
According to The International Working Group for Antibody Validation (IWGAV), suitable validation strategies for immunohistochemistry on formalin-fixed tissues include comparison with expression data obtained by another independent method.21 This is particularly straightforward for CPA1, because 3 independent RNA screening studies, including the FANTOM5 project,22,23 the Genotype-Tissue Expression (GTEx) project,24 and The Cancer Genome Atlas Programm (TCGA)25 (which are all summarized in the protein atlas26), demonstrate that CPA1 expression is strictly limited to pancreatic tissue. This is in perfect agreement with our immunohistochemistry data. A very high expression of CPA1 in pancreatic tissue is confirmed by our normal tissue analysis which in addition localizes the CPA1 protein to acinar cells and suggests that other cell types such as Islets of Langerhans and excretory ducts do not express CPA1 at measurable levels. The limitation of CPA1 expression to acinar cells is consistent with the function of CPA1, which is among the most important secretory enzymes.27 The strict limitation of CPA1 expression to all cases of pancreatic acinar cells obtained from 8 independent donors in our extensive normal tissue analysis demonstrates that CPA1 expression is indeed limited to this cell type and validates specificity and sensitivity of MSVA-601M. It is of note that negative results from RNA screening databases cannot rule out CPA1 expression in structures constituting only small fractions of the total cells of an organ. RNAs derived from small structures or rare cell types are largely underrepresented and thus potentially missed in RNA analyses. The absence of CPA1 immunostaining in any other normal tissue samples also argues against the potential cross-reactivity of our antibody in formalin-fixed tissues. Notably, the analysis of 76 different tissue categories ensures that a very large fraction of proteins that are being expressed in cells of adult humans have been exposed to the antibody and tested for cross-reactivity in this study.
The analysis of 12,274 human tumors, including samples from 132 different tumor types and subtypes, resulted in a similar “black and white” picture as seen in our normal tissue analysis and identified 12 strongly positive carcinomas and 12,262 entirely negative tumors. It is remarkable that 6 of these 12 CPA1 positive cancers had initially not been classified as ACC. Due to the high level of CPA1 expression in normal pancreatic acinar cells, the absolute specificity of our assay for pancreatic acinar cells among normal tissues, and the fact that these tumors were all located in the pancreas and showed high-level CPA1 expression, we challenged our initial diagnosis in these cases. In retrospect, 3 of these tumors (case #1, #5, and #6) were reclassified as definite ACC, since apart from CPA1 positivity, the tumors fulfilled at least some of the “classic” criteria for diagnosing an ACC. Two tumors (case #2 and #3) were reclassified as “probably ACC—see comment,” since these tumors apart from a strong CPA1 staining, did not fulfill the “classic” histomorphologic criteria, showed a focal weak or absent chymotrypsin immunostaining and a focal synaptophysin immunostaining. One tumor (case #4), initially classified as NEC, large cell variant, was newly diagnosed as probably MAEC, since parts of the tumor showed the typical morphology of a NEC (large cell variant) and part of the tumor showed moderate to strong CPA1 staining. Morphologic and immunohistochemical results of these tumors are shown in Figure 3. Reclassification of these tumors is not surprising, since ACCs can show several architectural patterns apart from the “classic” acinar pattern. The fact, that 5 of 11 ACCs and 1 mixed acinar endocrine carcinoma were initially not recognized reflects the well-known difficulties in correctly diagnosing this rare pancreatic cancer subtype. Even “experts” in gastrointestinal pathology will only rarely come across these tumors. In a retrospective study at John Hopkins only 14 ACCs were identified over a period of 18 years.28 Several other studies have emphasized on the difficulties in recognizing this rare subtype of pancreatic neoplasm.6,7 In a study by Basturk et al6 17 of 107 tumors initially diagnosed as poorly differentiated NECs were reevaluated and in retrospect diagnosed as pure ACC or mixed acinar-NEC.
FIGURE 3.

Morphologic and immunohistochemical findings of 6 cancers reclassified in this study.
Our data strongly suggest that CPA1 immunostaining should be broadly applied to newly diagnosed pancreatic tumors with “equivocal morphology” (ie, pancreatic tumors lacking a “classic” acinar pattern, without distinct PAS-positive cytoplasmatic zymogen granules and a disputable chymotrypsin staining) and that this examination has the potential to prevent relevant diagnostic errors. The high sensitivity for ACC detection seen in our study fits well with the findings with a recent study on CPA1 expression in pancreatic cancers where Said et al16 described strong diffuse CPA1 positivity in all 14 cases of ACC and 5 cases of MAEC analyzed. These authors, however, described a mostly focal or patchy CPA1 positivity to also occur in 2 of 20 ductal adenocarcinomas, 10 of 20 NETs, 2 of 20 mucinous cystic neoplasms, and 1 of 20 solid pseudopapillary tumor but still found a 95% specificity of CPA1 positivity for ACC if only patchy and diffuse staining was considered. While it cannot be excluded that some CPA1 positive non-ACCs also reflect diagnostic borderline cases in this study, it appears more likely that differences in the antibodies and protocols used for these studies are responsible for the higher rate of CPA1 positive non-ACCs. Our reclassification of tumors could be interpreted as a weakness of our study since this obviously could account for the high specificity of the marker. A comparison of the specificity of CPA 1 immunostaining before (99.5%) and after (100%) reclassification shows, however, that the specificity was not markedly impacted. The sensitivity is obviously unaffected and remains 100%.
It is of note that both the staining of large sections of 20 NETs and 17 ductal adenocarcinomas and the analysis of a pancreatic adenocarcinoma heterogeneity TMA containing up to 9 samples retrieved from different tumor blocks of 224 pancreatic cancers provided no evidence for even a minimal focal CPA1 immunostaining in non-ACCs. A weak CPA1 staining that involved both tumor cells and stroma cells that was occasionally seen immediately adjacent to highly CPA1 expressing normal pancreatic tissue was considered to be caused by diffusion of the highly abundant CPA1 from normal acinar cells that may have suffered some autolytic cell damage.
In summary, our data show the outstanding specificity of CPA1 immunostaining for acinar differentiation in the pancreas to such an extent that we suggest to include CPA1 immunostaining when faced with a pancreatic tumor with “equivocal morphology.” The data also demonstrate the strength of a large-scale tissue screening approach for clarifying the specificity and diagnostic utility of antibodies.
ACKNOWLEDGMENTS
The authors are grateful to Melanie Witt, Inge Brandt, Maren Eisenberg, and Sünje Seekamp for excellent technical assistance.
Footnotes
T.S.C. and F.J. contributed equally to this paper.
Conflicts of Interest and Source of Funding: The CPA1 antibody clone MSVA-601M was provided from MS Validated Antibodies GmbH (owned by a family member of G.S.). For the remaining authors none were declared.
Contributor Information
Ria Uhlig, Email: r.uhlig@uke.de.
Hendrina Contreras, Email: hendrina.contreras@uke.de.
Sören Weidemann, Email: s.weidemann@uke.de.
Natalia Gorbokon, Email: n.gorbokon@uke.de.
Anne Menz, Email: a.menz@uke.de.
Franziska Büscheck, Email: f.buescheck@uke.de.
Andreas M. Luebke, Email: luebke@uke.de.
Martina Kluth, Email: m.kluth@uke.de.
Claudia Hube-Magg, Email: c.hube@uke.de.
Andrea Hinsch, Email: a.hinsch@uke.de.
Doris Höflmayer, Email: d.hoeflmayer@uke.de.
Christoph Fraune, Email: c.fraune@uke.de.
Katharina Möller, Email: ka.moeller@uke.de.
Christian Bernreuther, Email: c.bernreuther@uke.de.
Patrick Lebok, Email: p.lebok@uke.de.
Guido Sauter, Email: g.sauter@uke.de.
Waldemar Wilczak, Email: w.wilczak@uke.de.
Jakob Izbicki, Email: izbicki@uke.de.
Daniel Perez, Email: d.perez@uke.de.
Stefan Steurer, Email: s.steurer@uke.de.
Eike Burandt, Email: e.burandt@uke.de.
Rainer Krech, Email: pathologie@ncid.net.
David Dum, Email: d.dum@uke.de.
Till Krech, Email: t.krech@uke.de.
Andreas Marx, Email: Andreas.Marx@klinikum-fuerth.de.
Ronald Simon, Email: r.simon@uke.de.
Sarah Minner, Email: s.minner@uke.de.
Frank Jacobsen, Email: f.jacobsen@uke.de.
Till S. Clauditz, Email: t.clauditz@uke.de.
REFERENCES
- 1.Niger M, Prisciandaro M, Antista M, et al. One size does not fit all for pancreatic cancers: a review on rare histologies and therapeutic approaches. World J Gastrointest Oncol. 2020;12:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klimstra DS, Adsay V. Acinar neoplasms of the pancreas—a summary of 25 years of research. Semin Diagn Pathol. 2016;33:307–318. [DOI] [PubMed] [Google Scholar]
- 3.Pokrzywa CJ, Abbott DE, Matkowskyj KA, et al. Natural history and treatment trends in pancreatic cancer subtypes. J Gastrointest Surg. 2019;23:768–778. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Hu G, Ma Y, et al. Acinar cell carcinoma of the pancreas presenting as diffuse pancreatic enlargement: two case reports and literature review. Medicine (Baltimore). 2017;96:e7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowery MA, Klimstra DS, Shia J, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16:1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol. 2012;36:1782–1795. [DOI] [PubMed] [Google Scholar]
- 8.Sigel CS, Klimstra DS. Cytomorphologic and immunophenotypical features of acinar cell neoplasms of the pancreas. Cancer Cytopathol. 2013;121:459–470. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary P. Acinar cell carcinoma of the pancreas: a literature review and update. Indian J Surg. 2015;77:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustafa S, Hruban RH, Ali SZ. Acinar cell carcinoma of the pancreas: a clinicopathologic and cytomorphologic review. J Am Soc Cytopathol. 2020;9:586–595. [DOI] [PubMed] [Google Scholar]
- 11.Thompson ED, Wood LD. Pancreatic neoplasms with acinar differentiation: a review of pathologic and molecular features. Arch Pathol Lab Med. 2020;144:808–815. [DOI] [PubMed] [Google Scholar]
- 12.Quiocho FA, Lipscomb WN. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. [DOI] [PubMed] [Google Scholar]
- 13.Hegyi E, Sahin-Toth M. Human CPA1 mutation causes digestive enzyme misfolding and chronic pancreatitis in mice. Gut. 2019;68:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet. 2013;45:1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemik O, Kemik AS, Sumer A, et al. Serum procarboxypeptidase A and carboxypeptidase A levels in pancreatic disease. Hum Exp Toxicol. 2012;31:447–451. [DOI] [PubMed] [Google Scholar]
- 16.Said S, Kurtin PJ, Nasr SH, et al. Carboxypeptidase A1 and regenerating islet-derived 1alpha as new markers for pancreatic acinar cell carcinoma. Hum Pathol. 2020;103:120–126. [DOI] [PubMed] [Google Scholar]
- 17.Steurer S, Riemann C, Buscheck F, et al. p63 expression in human tumors and normal tissues: a tissue microarray study on 10,200 tumors. Biomark Res. 2021;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weidemann S, Gagelmann P, Gorbokon N, et al. Mesothelin expression in human tumors: a tissue microarray study on 12,679 tumors. Biomedicines. 2021;9:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burandt E, Lubbersmeyer F, Gorbokon N, et al. E-Cadherin expression in human tumors: a tissue microarray study on 10,851 tumors. Biomark Res. 2021;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menz A, Bauer R, Kluth M, et al. Diagnostic and prognostic impact of cytokeratin 19 expression analysis in human tumors: a tissue microarray study of 13,172 tumors. Hum Pathol. 2021;115:19–36. [DOI] [PubMed] [Google Scholar]
- 21.Uhlen M, Bandrowski A, Carr S, et al. A proposal for validation of antibodies. Nat Methods. 2016;13:823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lizio M, Abugessaisa I, Noguchi S, et al. Update of the FANTOM web resource: expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47:D752–D758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lizio M, Harshbarger J, Shimoji H, et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman RL, Heath AP, Ferretti V, et al. Toward a shared vision for cancer genomic data. N Engl J Med. 2016;375:1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 27.Pandol SJ. The Exocrine Pancreas. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 28.Seth AK, Argani P, Campbell KA, et al. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg. 2008;12:1061–1067. [DOI] [PubMed] [Google Scholar]


