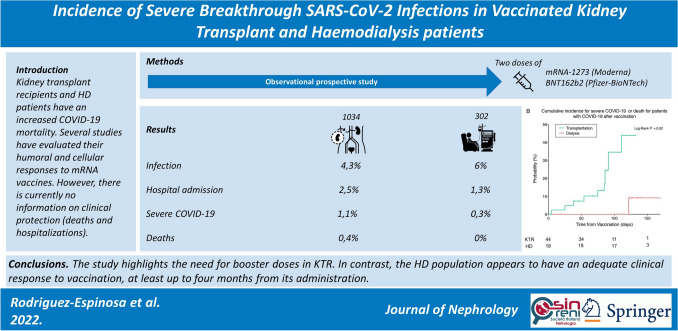
Abstract
Introduction
Given the increased COVID-19 observed in kidney transplant recipients (KTRs) and haemodialysis patients, several studies have tried to establish the efficacy of mRNA vaccines in these populations by evaluating their humoral and cellular responses. However, there is currently no information on clinical protection (deaths and hospitalizations), a gap that this study aims to fill.
Methods
Observational prospective study involving 1,336 KTRs and haemodialysis patients from three dialysis units affiliated to Hospital Clínic of Barcelona, Spain, vaccinated with two doses of mRNA-1273 (Moderna) or BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 mRNA vaccines. The outcomes measured were SARS-CoV-2 infection diagnosed by a positive RT-PCR fourteen days after the second vaccine dose, hospital admissions derived from infection, and a severe COVID-19 composite outcome, defined as either ICU admission, invasive and non-invasive mechanical ventilation, or death.
Results
Six per cent (18/302) of patients on haemodialysis were infected, of whom four required hospital admission (1.3%), only one (0.3%) had severe COVID-19, and none of them died. In contrast, 4.3% (44/1034) of KTRs were infected, and presented more hospital admissions (26 patients, 2.5%), severe COVID-19 (11 patients, 1.1%) or death (4 patients, 0.4%). KTRs had a significantly higher risk of hospital admission than HD patients, and this risk increased with age and male sex (HR 3.37 and 4.74, respectively).
Conclusions
The study highlights the need for booster doses in KTRs. In contrast, the haemodialysis population appears to have an adequate clinical response to vaccination, at least up to four months from its administration.
Graphical abstract
Keywords: COVID-19, mRNA SARS-CoV-2 vaccination, Haemodialysis, Kidney transplantation, Clinical efficacy
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), constitutes a significant health problem worldwide. Kidney transplant recipients (KTRs) and haemodialysis (HD) patients infected with SARS-CoV-2 have had worse outcomes than the general population in terms of more extended hospital stays, increased intensive care unit (ICU) admissions, and higher mortality [1–3]. Different vaccines have been developed to prevent infections and mitigate disease severity. Due to the increased risk, as well as to the previously known deficient immunological response of KTRs and HD patients to several classes of viral vaccines, such as hepatitis B virus and influenza [4–7], and to the fact that they induce more consistent protective immunity compared to other vector-based vaccines [8], mRNA vaccines were allocated to the end-stage kidney disease (ESKD) population in many countries.
Consequently, numerous articles analysed these patients’ immunological reactiveness to the two commercially available mRNA vaccines, the mRNA-1273 (Moderna) and the BNT162b2 (Pfizer-BioNTech), in terms of humoral (anti-S1-RBD IgG) and cellular (T-cell reactivity to N or S antigen) responses [9–12]. Most studies agree that over 80% of HD patients generate a humoral response [13] with marked reductions in antibody levels after three months [14]. In contrast, roughly 30% of KTRs are able to generate such response after two doses [10], with no available data on humoral maintenance among those with an initial immune response.
The pivotal clinical trials of both mRNA-1273 and BNT162b2 show 94–95% protection against SARS-CoV-2 infections [15, 16]. Real-life data on previously healthy vaccinated individuals regarding the protection against SARS-CoV-2 infection and COVID-19 severity are in accordance with these clinical trials [17, 18]. However, viral transmission has gained greater importance as new variants have emerged [19]. Currently, the vaccine’s success relies on the fact that SARS-CoV-2 breakthrough infections are less frequent and have much milder presentations (i.e., fewer hospital admissions and mortality) among the vaccinated than among those who are not [20, 21]. Nevertheless, there is no real-life data on mRNA vaccine efficacy in KTRs or in HD patients. Thus, this study aims to determine the incidence of SARS-CoV-2 infection and COVID-19 severity in these two sub-populations after two doses of an mRNA vaccine.
Methods
Study design
This retrospective observational study included KTRs from the Hospital Clínic of Barcelona and HD patients of this hospital and its three affiliated centres, Centre de Diàlisi i Recerca Aplicada Clínic, Fresenius Medical Care Roselló, and Diaverum Institut Hemodiàlisi Barcelona who had received two doses of either the BNT162b2 or mRNA-1273 vaccines between January and August 2021.
This study was conducted following the World Medical Association Declaration of Helsinki, national and local laws, and good clinical practice standards. The Institute's Committee on Human Research approved it.
Patient population
All KTRs or HD patients who received the complete vaccination schedule and were followed in the centres mentioned above were included. Exclusion criteria from the final analysis were patients younger than 18 years of age, those who received a non-mRNA vaccine (i.e., other than BNT162b2 or mRNA-1273 vaccine), those vaccinated after August 31st, 2021, patients with COVID-19 diagnosed within fourteen days after the second vaccine dose, patients with only one vaccine dose, and those with no available information about their vaccination status.
Demographic characteristics and comorbidities were obtained from electronic health records. Haemodialysis modality and duration data were collected in the HD group, whereas transplant modality, induction and maintenance immunosuppression data were retrieved for KTRs. Past history of SARS-CoV-2 infection before vaccination was defined as the presence of a positive RT-PCR before the administration of the first vaccine dose. The data were collected and treated following current legislation.
Clinical outcomes
The study outcomes were SARS-CoV-2 infection, hospital admission, and a severe COVID-19 composite outcome, defined as either ICU admission, invasive and non-invasive mechanical ventilation, or death due to COVID-19. Since complete immunization against SARS-CoV-2 is considered effective fourteen days after the second dose [16], only events after this period were considered during follow-up. The patients were censored at death or the last visit until August 31st, 2021. All transplant patients had at least one follow-up visit during the observation period and were followed-up at our centre. Patients were considered symptomatic for presenting any symptom attributable to COVID-19, regardless of severity.
Statistical analysis
Data are presented as mean ± standard deviation (SD) for parametric variables and median (interquartile range [IQR]) for the non-parametric ones. Differences in qualitative variables between groups were analysed with the χ2 test or Fisher’s exact test when one or more expected values were less than five or the data were very unequally distributed among the cells of the table. The normal distribution of the quantitative variables was tested with the Shapiro–Wilk test and Q–Q plots. The analysis of quantitative variables between two groups was made with the U-Mann Whitney test when non-normal or the independent Student’s t test when normally distributed. The Kaplan–Meier method with Log-Rank test was used for survival analysis. To evaluate the association between the defined predictor variables and COVID-19 infection, hospitalization for COVID-19, and severe COVID-19, a Cox univariate analysis was performed. Furthermore, to adjust for potential confounding covariates, a multivariable Cox regression analysis with two models was performed: Model 1 included sex and age as covariates; Model 2 included age, sex, dialysis vintage, hypertension, coronary artery disease, smoking, and diabetes. Due to the number of cases, Model 1 was applied only for COVID-19 infection (60 cases) and COVID-19 admission (30 cases), but for severe COVID-19 (12 cases). Model 2 was only applied for COVID-19 infection. Statistical analysis was performed using IBM SPSS Statistics 26.0 (SPSS, Inc; Chicago, Illinois) software for macOS. All tests were two-tailed, and the significance level was defined as a P-value < 0.05.
Results
Patient demographic characteristics
A total of 1,363 patients were initially included (305 HD patients and 1,058 KTR). Among them, 27 patients were excluded because they had received a non-mRNA COVID-19 vaccine (1), had been vaccinated after August 31st, 2021(14 patients), had received a diagnosis of SARS-CoV-2 infection within 14 days after the second vaccine dose (6), had received only one vaccine dose (3), or because of a lack of available information about their vaccine status (3 patients) (Fig. 1).
Fig. 1.
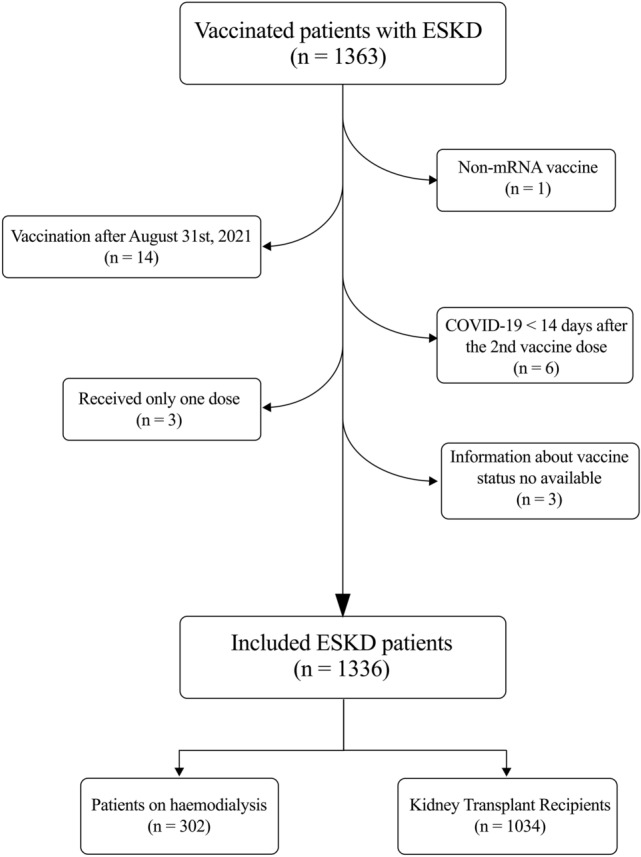
Flowchart of the included patients. ESKD, End-Stage Kidney Disease; COVID-19, Coronavirus Disease 2019
Among the 1336 ESKD patients finally included, 302 patients were on dialysis (94% haemodiafiltration, 6% haemodialysis), and 1034 were KTRs (88% had received a kidney transplant, 9% a simultaneous pancreas-kidney transplant, and 3% were other multiorgan transplant recipients). For the overall population, the mean follow-up time was 122 ± 39 days. When compared to KTRs, dialysis patients were older (73.07 ± 14.33 vs 58.71 ± 13.15, P < 0.001), more of them had diabetes (42% vs 23%, P < 0.001), smoking habit (28% vs 18%, P < 0.001) and coronary heart disease (28% vs 18%, P < 0.001). In contrast, KTRs had higher BMI (25.10 ± 5.56 vs 26.00 ± 4.80 kg/m2, P = 0.007) and more of them had hypertension (79% vs 67%, P < 0.001). Table 1 summarises the baseline characteristics of the HD patients and KTRs included in the study.
Table 1.
Patient baseline characteristics
| Dialysis patients (n = 302) | Kidney transplant recipients (n = 1034) | P-value | |
|---|---|---|---|
| Age (years) | 73.07 ± 14.33 | 58.71 ± 13.15 | < 0.0001 |
| Gender (male) | 197 (65) | 668 (65) | 0.81 |
| Ethnicity | 0.01 | ||
| Caucasian | 271 (91) | 965 (93) | |
| Hispanic | 10 (3) | 34 (3) | |
| Asian | 4 (1) | 15 (2) | |
| African American | 4 (1) | 7 (1) | |
| Arabic | 13 (4) | 12 (1) | |
| BMI (kg/m2) | 25.10 ± 5.56 | 26.00 ± 4.80 | 0.007 |
| Diabetes (yes) | 127 (42) | 233 (23) | < 0.001 |
| Smoking habit (yes) | 84 (28) | 187 (18) | < 0.001 |
| Hypertension (yes) | 204 (67) | 814 (79) | < 0.001 |
| Coronary Heart Disease (yes) | 77 (26) | 126 (12) | < 0.001 |
| Dialysis modality | – | ||
| Haemodiafiltration | 286 (94) | – | |
| Haemodialysis | 16 (5) | – | |
| Transplant modality | – | ||
| KTA | – | 911 (88) | |
| SPK | – | 88 (9) | |
| Other | – | 35 (3) | |
| Dialysis vintage (months) | 35 [19–69] | 20 [6–43] | 0.23 |
| Transplant vintage (months) | 0.5 [0–96] | 106 [47–193] | < 0.001 |
| Previous KT (any) | 13 (16) | 219 (21) | 0.32 |
| Induction IS | |||
| No | – | 205 (19) | |
| Basiliximab | – | 361 (34) | |
| Thymoglobulin | – | 492 (47) | |
| Maintenance IS | |||
| TAC + MPA | – | 624 (59) | |
| TAC + mTORi | – | 255 (24) | |
| Other | – | 179 (17) | |
| Pre-vaccine COVID-19 (yes) | 27 (9) | 81 (8) | 0.55 |
| COVID-19 vaccine | < 0.001 | ||
| mRNA-1273 | 194 (64) | 1,027 (99) | |
| BNT162b2 | 108 (36) | 7 (1) |
Data are mean ± SD, median [IQR] or n (%), unless otherwise specified. BMI, Body Mass Index; KTA, Kidney Transplantation Alone; SPK, Simultaneous Pancreas-Kidney; KT, Kidney Transplantation; IS, Immunosuppression; TAC, Tacrolimus; MPA, Mycophenolate; mTORi, mTOR inhibitors; COVID-19, Coronavirus Disease 2019
COVID-19 breakthrough infections after vaccination
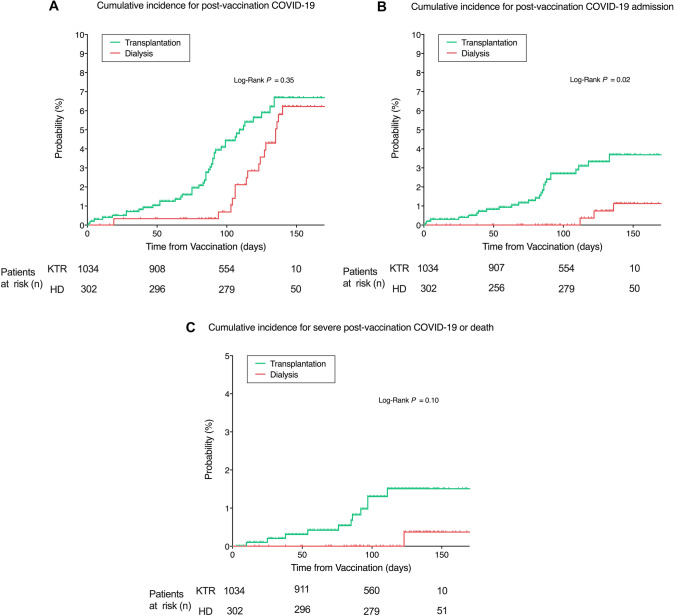
Most of the patients were vaccinated with the mRNA-1273 vaccine (64% vs 99% of the dialysis and transplant patients, respectively, P < 0.001). After vaccination, a total of 18 HD patients (6.0%) and 44 KTRs (4.3%) were diagnosed with COVID-19 after a mean time from vaccination of 136 ± 26 vs 99 ± 38 days for dialysis and transplant recipients, respectively. Of these patients, 14 HD patients (5%) and 18 KTRs (2%) were managed as outpatients, with 4 and 1 of them being asymptomatic, respectively. The incidence of COVID-19 (regardless of the severity) at 50, 100, and 150 days after vaccination was 0.33% vs 1.03%, 0.69% vs 4.43%, and 6.22% vs 6.68% for KTRs and HD patients, respectively, without differences between groups (P = 0.35) (Fig. 2A).
Fig. 2.
Cumulative incidence of COVID-19 after vaccination (total population). A Cumulative incidence of COVID-19 after vaccination for kidney transplant and dialysis patients. B, Cumulative incidence of hospital admissions for COVID-19 infection in kidney transplant and dialysis patients; C Cumulative incidence of severe COVID-19 or death in vaccinated kidney transplant and dialysis patients. Time is calculated since vaccine protection (+ 14 days after the second dose). KTR, Kidney Transplant Recipients. HD Haemodialysis
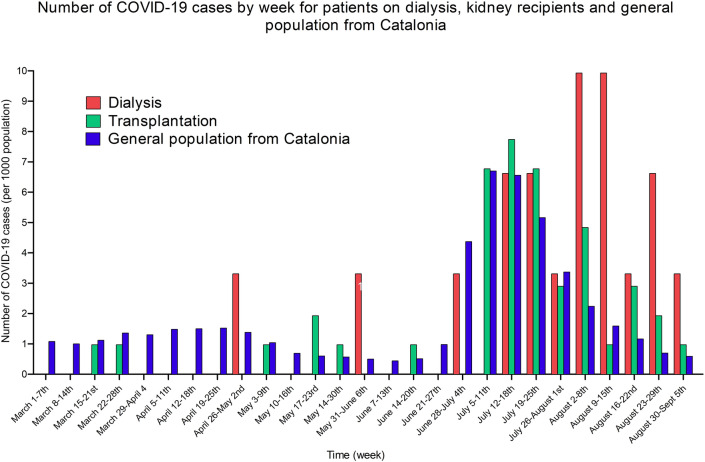
The observed incidence per 1000 population of COVID-19 in vaccinated HD patients and KTRs was compared with the reported incidence of COVID-19 in the general population from Catalonia (Fig. 3) [22]. Although a sustained number of cases were reported during the study period for the general population in Catalonia, the highest incidence of COVID-19 was observed between June 28th, 2021, and August 22nd, 2021, during the fifth wave of the disease in this territory. This peak of COVID-19 cases followed the peak of cases observed for dialysis patients and KTRs, with similar incidences (although a higher number of cases was reported for dialysis patients at the beginning of August 2021).
Fig. 3.
Number of COVID-19 cases by week for patients on dialysis, kidney transplant recipients and general population from Catalonia (per 1000 population)
Hospital admission for COVID-19 after vaccination
Among the analysed patients, 4 vaccinated patients on dialysis (1.3% of the total population, 22.2% of infected patients) and 26 KTRs (2.5% of the total population, 59.1% of infected patients) were admitted to the hospital due to COVID-19. The mean time from vaccination to hospital admission was 140 ± 35 vs 68 ± 36 days for HD patients and KTRs, respectively. The incidence of COVID-19 admission at 50, 100, and 150 days after vaccination was significantly higher for transplant recipients (0.82% vs 0%, 2.69% vs 0%, and 3.69% vs 1.13% at 50, 100, and 150 days for transplant and dialysis patients, respectively, P = 0.02) (Fig. 2B). Similar results were observed when only patients with COVID-19 after vaccination were considered, with an incidence of hospital admission due to COVID-19 of 19% vs 0%, 59% vs 0, and 78% vs 37% at 50, 100, and 150 days after vaccination for HD patients and KTRs, respectively (P < 0.001) (Fig. 4A). There was no correlation on multivariable analysis between hospital admissions and baseline characteristics.
Fig. 4.
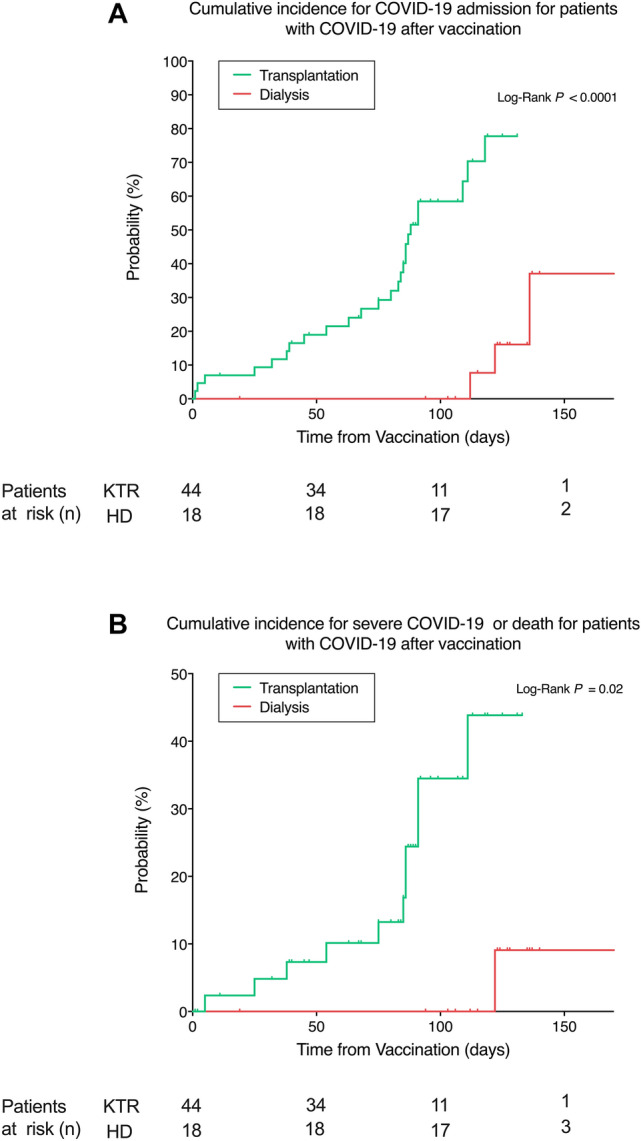
Cumulative incidence for hospital admission and severe COVID-19 (infected population). A Cumulative incidence for COVID-19 admission for kidney transplant and dialysis patients with COVID-19 after vaccination. B Cumulative incidence for severe COVID-19 or death for kidney transplant and dialysis patients after vaccination. Time is calculated since vaccine protection (+ 14 days after the second dose). KTR kidney transplant recipients, HD haemodialysis
Incidence of severe COVID-19 or death after vaccination
One HD patient (0.3% of total population, 5.5% of the infected patients) and eleven KTRs (1.1% of total population, 25.0% of infected patients) from the overall vaccinated population exhibited severe COVID-19 requiring ICU admission. Among the KTRs admitted to the ICU, four (0.4% of the total population, 9.1% of infected patients) died while no deaths were observed in the dialysis group. The incidence of the severe COVID-19 composite outcome at 50, 100, and 150 days was 0.3% vs 0%, 1.3% vs 0%, and 1.5% vs 0.4% for KTRs and HD patients, respectively (P = 0.10) (Fig. 1C). Nevertheless, when considering the incidence of the severe COVID-19 composite outcome among infected individuals alone, results were significantly higher in KTRs for the three analysed time-points (7.3% vs 0%, 34.5% vs 0%, and 43.8% vs 9.1% at 50, 100 and 150 days for KTRs and HD patients, respectively, P = 0.02) (Fig. 4B). There was no correlation on multivariable analysis between severe COVID-19 or death and baseline characteristics.
Factors associated with risk of infection and clinical outcomes
There was a significant increased risk for hospital admission in the multivariable linear regression model for KTRs in comparison to HD patients both for the unadjusted model (HR 3.37 [95% CI 1.21–10.10], p = 0.03) and the adjusted one for increased age and male sex (HR 4.75, [95% CI 1.23–18.35], p = 0.02). The second model of sex, age, dialysis or transplant vintage, history of hypertension, coronary artery disease, and smoking habit did not correlate with the risk of SARS-CoV-2 infection (p = 0.25) and the number of events was too small to perform any further analysis (Table 2).
Table 2.
Multivariable regression analysis of potential associated factors with SARS-CoV-2 infection and COVID-19 severity in kidney transplant recipients and haemodialysis patients
| KTR vs HD | SARS-CoV-2 infection | P-value | Hospital admission | P-value | Severe COVID-19 | P-value |
|---|---|---|---|---|---|---|
| Unadjusted | HR 1.30 [95% CI 0.74–2.30] | 0.36 | HR 3.37 [95% CI 1.21–10.10] | 0.03 | HR 4.58 [95% CI 0.58–35.70] | 0.39 |
| Adjusted Model 1 | HR 1.19 [95% CI 0.63–2.26] | 0.59 | HR 4.75 [95% CI 1.23–18.35] | 0.02 | n.a | |
| Adjusted Model 2 | HR 1.50 [95% CI 0.76–2.98] | 0.25 | n.a | n.a |
Model 1 included as covariates: sex, age. Model 2 included as covariates: sex, age, dialysis or transplant vintage, diabetes, smoking habit, coronary heart disease and hypertension. KTR, kidney transplant recipients; HD, haemodialysis; SARS-CoV-2, severe acute respiratory coronavirus 2; COVID-19, coronavirus disease 2019
Discussion
Among the affected population during COVID-19 waves, both KTRs and HD patients were the two groups with higher susceptibility and poorer outcomes. Our results show that there was a remarkable overall decline in the incidence of SARS-CoV-2 infection, both in kidney transplant and haemodialysis patients who received two doses of either mRNA vaccine, in comparison to the incidence reported in the same population during the first wave of COVID-19 in 2020 [2, 3, 23].
The reported data are in accordance with the published reduced infection rates seen among the general population [17, 24], with the peak incidence of cases in our KTR cohort matching the peak number of cases reported in Catalonia [22], whereas the HD population appears to have peaked four weeks later, though we have not found a plausible cause for this temporal shift23. The main driving factor for the observed decrease in infection is probably the widespread vaccination process applied to all adult citizens in Spain. However, the improved understanding of the disease’s behaviour, proper confinement, and personal sanitary measures may have also lowered the incidence of the disease despite the vaccines’ lessened capacity to eradicate infections among the exposed vaccinated general population since the dawn of new SARS-CoV-2 variants [18, 20].
We observed a tendency towards a higher incidence of COVID-19 in HD patients than in KTRs, albeit not a significant one. This may be due to the capacity of KTRs to adopt proper confinement and isolation measures while HD patients can not because of their every-other-day contact with the healthcare system and use of public transportation. Moreover, there can be a bias regarding the detection of a higher number of cases in HD patients because of the routine monthly SARS-CoV-2 RT-PCR screening performed in our centres, which may have increased the COVID-19 diagnosis at the expense of asymptomatic cases [23]. In contrast, the exact number of asymptomatic transplant recipients is unknown, as, during the study period, they were only tested with a nasopharyngeal swab RT-PCR test if they presented compatible symptoms or had been in close contact with an infected individual.
Even though a lower infection rate remains an important outcome, physicians and health authorities believe that the real success of vaccination lies in obtaining a reduction in significant clinical outcomes such as fewer and shorter hospital admissions, as well as less severe cases with fewer ICU admissions and lower mortality associated with COVID-19. The results obtained in our study show an overall improvement in all these clinical outcomes in comparison to infected individuals, from the same population cohort, during the first wave of the pandemic [2, 3]; however, these improved outcomes were mainly driven by results seen in HD patients, despite them being significantly older, predominantly male, and with more comorbidities than their transplanted counterparts. The vaccinated HD patients who required hospital admission for COVID-19 made up only 22.2%, with only one case of severe disease (5.5%) and no deaths. In comparison, during the the first wave of the disease [2], 85% of infected HD patients were admitted, and 38% of them died. Contrarily, KTRs continued to have a similarly high number of hospital admissions (59.1%) and mortality (9.1%) in comparison with 2020 (79% and 6%, respectively) [3] (Table 3). The observed difference could be secondary to the previously seen disparity in the immune response reported between these populations [9, 10]. All the published data on the subject concur that larger proportions of HD patients generate anti-S1-RBD IgG antibodies, and they do it with higher titres than KTRs. The higher amount of anti-S1-RBD IgG titres that are present in HD subjects has probably prevented COVID-19-related complications in this group of patients, as they are a surrogate for viral neutralization capacity [25]. However, the length of antibody persistence remains uncertain and is perhaps less than that of the general population, as we must consider that they still have a reduced immune capacity because of [4, 6], although not as reduced as in patients receiving chronic immunosuppression. Moreover, we found that sex and age were associated with an increased risk of hospital admission but not of infection or death. Since our cohort was not powered to find any predictors and the number of events was low, no definitive conclusions can be derived from this analysis.
Table 3.
Number of detected SARS-CoV-2 infection cases and their clinical outcomes in kidney transplant recipients and haemodialysis patients before and after complete mRNA vaccination
| Haemodialysis patients | Kidney transplant recipients | |||
|---|---|---|---|---|
| Pre-vaccination (1st wave) [2] | Post-vaccination (5th wave) | Pre-vaccination (1st wave) [3] | Post-vaccination (5th wave) | |
| SARS-CoV-2 infection | 34/372 (9%) | 18/302 (6%) | 33/1043 (3.2%) | 44/1032 (4.3%) |
| Hospital admissions | 29/34 (85.3%) | 4/18 (22.2%) | 26/33 (78.8%) | 26/44 (59.1%) |
| Severe COVID-19 | 8/34 (23.5%) | 1/18 (5.5%) | 13/33 (39.4%) | 11/44 (25%) |
| Deaths | 13/34 (38.2%) | 0/18 | 2/33 (6%) | 4/44 (9.1%) |
Our study has some limitations. Firstly, it is a retrospective study and information about anti-S1-RBD IgG in all patients after vaccination was not available. It would have provided valuable data to determine whether protection comes from the mere presence or a specific titre of antibodies. Also, the demographic data and comorbidities of HD patients and KTRs are not homogeneous, and thus, comparisons between these two groups should be taken cautiously. Another difference between the two populations that has to be taken into account is the higher prevalence of vaccination with BNT162b2 in HD patients, while almost all KTRs were vaccinated with mRNA-1273.
In conclusion, our results suggest that mRNA SARS-CoV-2 vaccines confer close to complete protection from severe COVID-19 episodes in HD patients, at least during the four months following complete (two doses) mRNA vaccination. The finding of numerous breakthrough severe infections in KTRs claims for booster doses in this delicate population. Nevertheless, more studies are needed to be able to recommend more specific time frames for these dosing schedules, or if more than three doses are required.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Diana Rodríguez-Espinosa and Enrique Montagud-Marrahi are co-first authors.
José Jesús Broseta and David Cucchiari have contributed equally to this manuscript.
Contributor Information
José Jesús Broseta, Email: jjbroseta@clinic.cat.
David Cucchiari, Email: cucchiari@clinic.cat.
References
- 1.Gandolfini I, Delsante M, Fiaccadori E, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1941–1943. doi: 10.1111/AJT.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broseta JJ, Rodríguez-Espinosa D, Cuadrado E, et al. SARS-CoV-2 infection in a spanish cohort of CKD-5D patients: Prevalence, clinical presentation, outcomes, and de-isolation results. Blood Purif. 2020 doi: 10.1159/000510557. [DOI] [PubMed] [Google Scholar]
- 3.Montagud-Marrahi E, Cofan F, Torregrosa J-V, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2020;26:3. doi: 10.1111/ajt.15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eleftheriadis T, Antoniadi G, Liakopoulos V, et al. Disturbances of acquired immunity in haemodialysis patients. Semin Dial. 2007;20:440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 6.Anding K, Gross P, Rost JM, et al. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol Dial Transplant. 2003;18:2067–2073. doi: 10.1093/ndt/gfg330. [DOI] [PubMed] [Google Scholar]
- 7.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza A H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis. 2011;52:248–256. doi: 10.1093/CID/CIQ104. [DOI] [PubMed] [Google Scholar]
- 8.Windpessl M, Bruchfeld A, Anders H-J et al (2021) COVID-19 vaccines and kidney disease. Nat Rev Nephrol 17:5 17:291–293. 10.1038/s41581-021-00406-6 [DOI] [PMC free article] [PubMed]
- 9.Broseta JJ, Rodríguez-Espinosa D, Rodríguez N, et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to haemodialysis patients. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti–SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99:1487–1489. doi: 10.1016/J.KINT.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. doi: 10.1111/AJT.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacson E, Argyropoulos C, Manley H et al (2021) Immunogenicity of SARS-CoV-2 vaccine in dialysis. J Am Soc Nephrol. 10.1681/ASN.2021040432 [DOI] [PMC free article] [PubMed]
- 14.Broseta JJ, Rodríguez-Espinosa D, Bedini JL, et al. Antibody maintenance 3 months after complete mRNA COVID-19 vaccination in haemodialysis. Nephrol Dial Transplant. 2014;16:518–524. doi: 10.1093/NDT/GFAB272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden LR, el Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/nejmoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenforde MW (2021) Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults — United States, March–July 2021. MMWR Morbidity and Mortality Weekly Report 70:1156–1162. 10.15585/MMWR.MM7034E2 [DOI] [PMC free article] [PubMed]
- 18.Bernal JL, Andrews N, Gower C et al (2021) Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. 385:585–594. 10.1056/NEJMOA2108891 [DOI] [PMC free article] [PubMed]
- 19.Charmet T, Schaeffer L, Grant R, et al (2021) Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: results from a nationwide case-control study in France. Lancet Regional Health Eur 8:100171. 10.1016/J.LANEPE.2021.100171 [DOI] [PMC free article] [PubMed]
- 20.Nanduri S (2021) Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morbidity and Mortality Weekly Report 70:1163–1166. 10.15585/MMWR.MM7034E3 [DOI] [PMC free article] [PubMed]
- 21.Fowlkes A (2021) Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance — Eight U.S. Locations, December 2020–August 2021. MMWR Morbidity and Mortality Weekly Rep 70:1167–1169. 10.15585/MMWR.MM7034E4 [DOI] [PMC free article] [PubMed]
- 22.Dades actualitzades SARS-CoV-2. Agència de Qualitat i Avaluació Sanitàries de Catalunya (AQuAS). https://aquas.gencat.cat/ca/actualitat/ultimes-dades-coronavirus. Accessed 5 Oct 2021
- 23.Rodríguez-Espinosa D, Broseta JJ, Cuadrado E, Maduell F. Prevalence of COVID-19 infection in haemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:2967–2967. doi: 10.1681/ASN.2020081193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajema KL (2021) Effectiveness of COVID-19 mRNA Vaccines Against COVID-19–Associated Hospitalization—Five Veterans Affairs Medical Centers, United States, February 1–August 6, 2021. MMWR Morbidity and Mortality Weekly Report 70:1294–1299. 10.15585/MMWR.MM7037E3 [DOI] [PMC free article] [PubMed]
- 25.Irsara C, Egger A, Prokop W, et al (2021) Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clinical Chemistry and Laboratory Medicine (CCLM) 0:2021.02.17.21251907. 10.1101/2021.02.17.21251907 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.