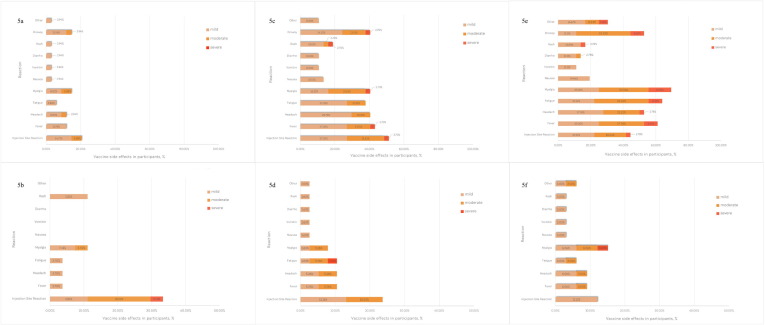
Fig. 5.
Adverse events on day 1 and day 7 after AZD1222 vaccination among participants who previously received two doses of CoronaVac. Figure 5a; Adverse events on day 1 in ID1 group. Figure 5b; Adverse events on day 7 in ID1 group. Figure 5c; Adverse events on day 1 in ID2 group. Figure 5d; Adverse events on day 7 in ID2 group. Figure 5e; Adverse events on day 1 in IM group. Figure 5f; Adverse events on day 7 in IM group. ID1, 20% intradermal fractional dose; ID2, 40% intradermal fractional dose. IM, intramuscular standard dose.