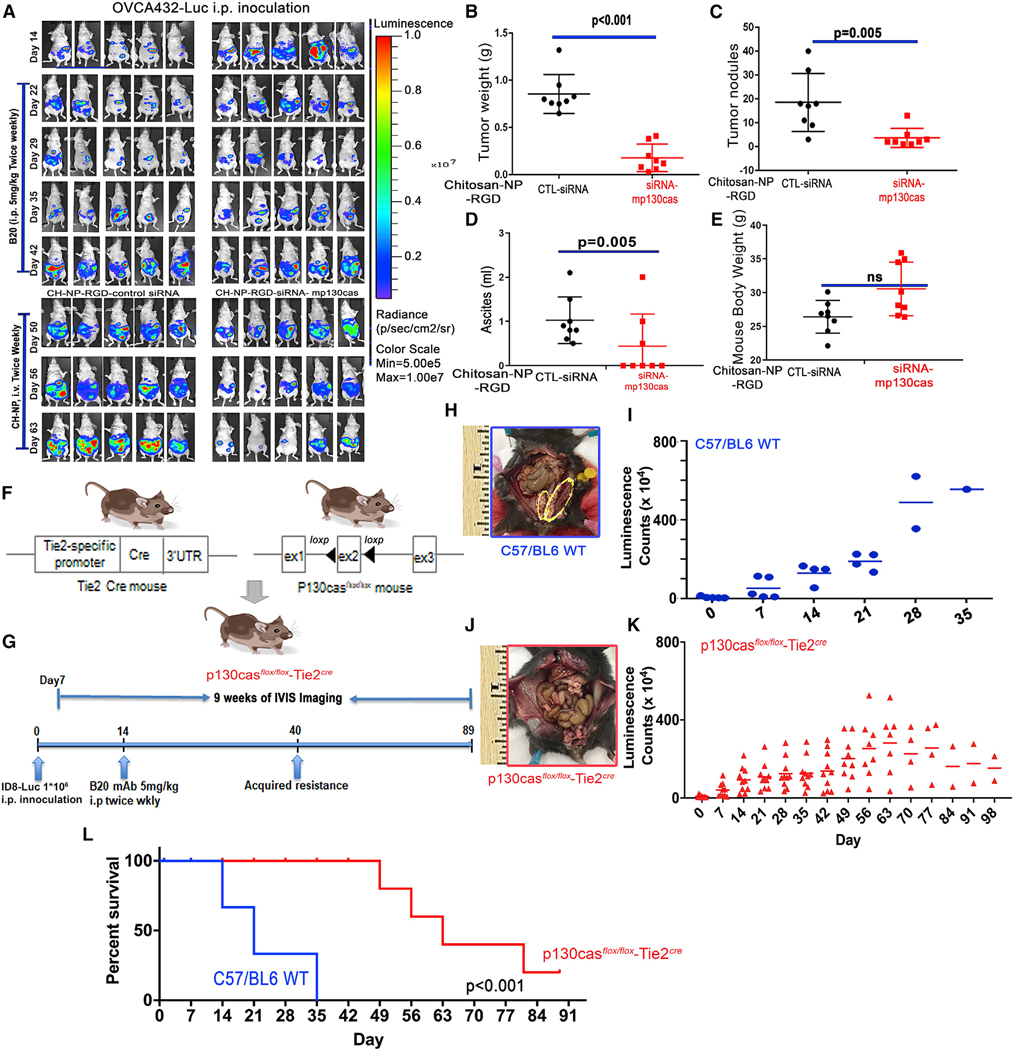
Figure 5. Ablation of vascular p130cas delays progression of tumors with adaptive resistance to AVA therapy.
(A) Mouse models of adaptive resistance to the anti-VEGF antibody (B20) were established using an orthotopic model of OVCA432 cells labeled with luciferase (OVCA432-luc). Mice with adaptive resistance (e.g., after development of ‘‘breakthrough’’ disease) were randomized and treated with CH-NP-RGD-CTL siRNA or CH-NP-RGD-siRNA-mp130cas.
(B–E) The (B) tumor weight, (C) number of tumor nodules, (D) volume of ascites, and (E) body weight of each mouse were recorded at necropsy. Data are expressed as mean ± SD: (B) p < 0.001, (C) p = 0.005, (D) p = 0.05, and (E) p = 0.262, determined by two-tailed, nonparametric t test.
(F) Schematic of the excision of exon 2 from the p130casflox/flox allele by Cre recombinase expressed in Tie2Cre mice to generate p130casflox/floxTie2Cre mice.
(G) Schematic of the dosing and in vivo bioluminescence imaging regimen used to establish the syngeneic ID8 ovarian tumor mouse model with adaptive resistance to B20 treatment.
(H and J) Representative gross images from C57/BL6 (H, on day 35) or p130casflox/floxTie2Cre (J, on day 98) mice. Syngeneic ID8 tumors, which were implanted surgically into the left ovary of each mouse, are labeled with yellow ovals (H).
(I and K) ID8 tumor burden, demonstrated with bioluminescence in C57/BL6 or p130casflox/floxTie2Cre mice.
(L) Kaplan-Meier survival plot of C57/BL6 and p130casflox/floxTie2Cre mice bearing intra-ovarian ID8 tumors and receiving B20 treatments; p < 0.001, log rank test.