Abstract
OBJECTIVES
There are limited data available on the height of the ventricular component of the septal deficiency (VSD) in patients undergoing complete atrioventricular septal defect (CAVSD) repair. VSD height may influence optimal choice of repair strategy with potential consequences for long-term outcomes. We aimed to measure VSD height using 2-dimensional echocardiography and review its association with postoperative outcomes.
METHODS
We retrospectively reviewed the preoperative echocardiograms of 45 consecutive patients who underwent CAVSD repair between May 2010 and December 2015 at a single centre. VSD height and left ventricular length on the four-chamber view were measured. Demographic details and early and late outcomes including reoperation and long-term survival were studied.
RESULTS
Twenty patients underwent modified single-patch repair and 25 patients underwent double-patch repair of CAVSD. VSD height in the modified single-patch group ranged from 4.2 to 11.7 mm and in the double-patch group ranged from 5.1 to 14.9 mm. Nine patients had a deep ‘scoop’ with a VSD height of >10 mm, (7 double patch, 2 modified single patch). VSD height did not correlate with a specific Rastelli classification. There was no significant difference in the VSD height (P = 0.51) or the VSD height-to-left ventricular length ratio (P = 0.43) between the 2 repair groups. There was no 30-day mortality. Eight patients required reoperation; however, VSD height was not a significant predictor of reoperation (hazard ratio 0.95, 95% confidence interval 0.69–1.33; P = 0.08).
CONCLUSIONS
There was no correlation between VSD height and risk of reoperation after CAVSD repair. A deep ventricular scoop is uncommon in CAVSD patients.
Keywords: Complete atrioventricular septal defect, Complete atrioventricular canal, Atrioventricular septal defect
Recent studies [1] have demonstrated no significant difference in long-term outcomes based on the surgical repair technique following repair of complete atrioventricular septal defect (CAVSD).
INTRODUCTION
Recent studies [1] have demonstrated no significant difference in long-term outcomes based on the surgical repair technique following repair of complete atrioventricular septal defect (CAVSD). The 2 most commonly employed repair strategies are the modified single-patch (MSP) and the double-patch (DP) techniques. The DP technique has been the most extensively utilized technique and involves placement of separate atrial and ventricular septal patches. In contrast, the MSP technique, which was developed by Wilcox et al. [2] and Nunn [3, 4] independently in the 1990s, has rapidly grown in popularity due to its relative technical ease, as there is no requirement to size a ventricular septal patch (as with the DP technique) or to divide bridging leaflets (as in the classical single-patch technique). However, a large ventricular component to the septal deficiency, especially with a deep ventricular septal defect (VSD), continues to be a concern for some surgeons when considering whether to perform the MSP technique for CAVSD repair [5]. There has long been concern both for the propensity to develop left ventricular outflow tract obstruction (LVOTO) and for the potential impact on left atrioventricular valve (LAVV) function with the MSP technique [6–10] due to the approximation of the valvar leaflets to the ventricular septal crest. Although previous studies [1] demonstrate comparable outcomes with both MSP and DP techniques, it is impossible to control for surgeon preference in choosing repair technique based on subtleties of CAVSD anatomy such as VSD height and depth of septal scoop.
Echocardiography is an essential part of the preoperative assessment of CAVSD patients prior to repair [11] and its evolution over time has revolutionized the diagnosis and management of patients with CAVSD [12]. Previous echocardiographic studies have demonstrated no significant difference in the LAVV annulus size, tenting height, size of vena contracta and left ventricular outflow tract (LVOT) volumes in patients undergoing the DP and MSP technique [13, 14]. However, there are currently limited data available in the literature regarding the VSD height or ‘scoop’ depth [5, 15] and its correlation with the chosen surgical technique and clinical outcomes. These anatomical data are important as the depth of the septal ‘scoop’ may influence the likelihood of LVOTO or LAVV regurgitation following different repair strategies [5]. This study aimed to determine the prevalence of a deep ventricular ‘scoop’ in patients undergoing CAVSD repair and review its association with postoperative outcomes and repair technique (DP vs MSP).
PATIENTS AND METHODS
Patient population
A total of 47 consecutive patients who had undergone biventricular surgical repair of CAVSD between May 2010 and December 2015 were identified from institutional databases located at The Children’s Hospital at Westmead, Sydney, Australia. Preoperative echocardiograms were available for analysis in 45 patients who were included in the study. Patients with an associated diagnosis of tetralogy of Fallot and other conotruncal defects were excluded. The study was approved by the Sydney Children’s Hospital Network Human Research Ethics Committee (HREC/16/SCHN/216) and the need for individual patient consent was waived.
Two-dimensional echocardiographic assessment
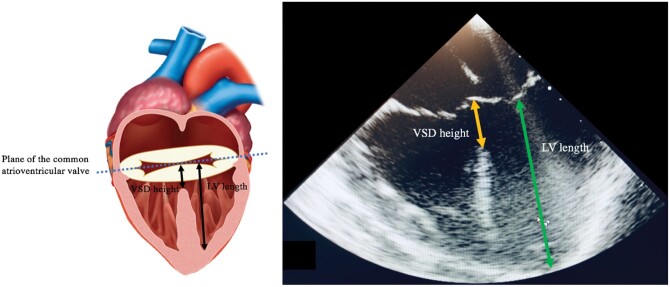
Two separate operators independently measured the size of the ventricular component of the septal deficiency (VSD height) and the left ventricular (LV) length on the four-chamber view at end-diastole (Fig. 1). The VSD height was defined as the distance from the deepest point of the ventricular component at the level of the atrioventricular junction to the intersection between the atrioventricular valve components. Since the size of the hearts varied within this study, the measurements were standardized by measuring the LV length, as a marker of LV size. LV length was defined as the distance from the LV apex to the intersection of the atrioventricular valve components at the atrioventricular junction. The ratio of the VSD height to LV length was then calculated for comparison. Since the LV apex may not be included in the four-chamber view, an assessment of foreshortening [where the 2-dimensional (2D) ultrasound plane does not cut through the true apex] was also made due to the risk of underestimating the LV dimension on this view. The mean values of these 2 measurements were then used as final discrete values.
Figure 1:
Demonstration of measurements of the VSD height and LV length measured using 2-dimensional echocardiography on four-chamber view. LV: left ventricular; VSD: ventricular septal defect.
Surgical technique
The procedural details for repair of CAVSD have been reported previously [16]. Briefly, all CAVSD repair operations were performed via a median sternotomy with aortobicaval cannulation and moderate hypothermia with either the DP or modified single-patch (MSP) technique depending on the surgeon preference. Patient follow-up data were obtained through hospital, clinic, and cardiologist medical records. Overall median follow-up duration was 4.2 (interquartile range 2.8–5.8) years.
Statistical analyses
Continuous variables were described as median and range and categorical variables were compared using the Pearson’s χ2 or Fisher’s exact test. Differences between continuous variables were analysed using the Mann–Whitney U-test for 2 samples. Risk factors for time to first reoperation were modelled using Cox regression modelling with the associated hazard ratio (HR) estimated. P-value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS 25.0 for Mac (SPSS, Chicago, IL, USA).
RESULTS
Patient characteristics
Of the 45 patients identified, 20 underwent MSP and 25 underwent DP repair of CAVSD. Table 1 lists the patient demographic details with no significant differences demonstrated between the 2 groups based on repair technique. The median age at the time of repair was 4.2 (interquartile range 2.3–5.7) months for MSP and 3.5 (interquartile range 2.5–4.3) months for the DP group with no significant difference between groups (Table 1, P = 0.52). The zone of apposition was closed in all MSP cases and in 23/25 (92%) of DP cases. The cardiopulmonary bypass and aortic cross-clamp times were significantly shorter in the MSP group compared with the DP group (Table 1).
Table 1:
Patient and operative characteristics of complete atrioventricular septal defect repair
| Characteristics | MSP (n = 20) | DP (n = 25) | P-value |
|---|---|---|---|
| Age at AVSD repair (months), median (IQR) | 4.2 (2.3–5.7) | 3.5 (2.5–4.3) | 0.52 |
| Weight (kg), median (IQR) | 4.5 (3.8–5.5) | 4.5 (3.6–5.2) | 0.59 |
| Female gender, n (%) | 11 (55) | 11 (44) | 0.46 |
| Down syndrome, n (%) | 9 (45) | 10 (40) | 0.74 |
| Associated cardiovascular anomalies, n (%) | |||
| Coarctation of aorta | 1 (5) | 2 (8) | 0.69 |
| DOLAVV | 0 | 3 (12) | 0.11 |
| Single papillary muscle | 0 | 3 (12) | 0.11 |
| LVOTO | 0 | 0 | |
| Preoperative ECHO LAVVR ≥ 3 | 3 (15) | 2 (8) | 0.46 |
| Rastelli class, n (%) | 0.49 | ||
| A | 8 (40) | 12 (48) | |
| B | 1 (5) | 0 | |
| C | 11 (55) | 13 (52) | |
| Prior PAB, n (%) | 4 (20) | 2 (8) | 0.24 |
| Management of zone of apposition, n (%) | 0.20 | ||
| Complete closure | 20 (100) | 23 (92) | |
| Left open | 0 | 2 (8) | |
| Cardiopulmonary bypass time (min), mean ± SD | 126 ± 45 | 167 ± 57 | 0.001 |
| Aortic cross-clamp time (min), mean ± SD | 88 ± 38 | 132 ± 50 | 0.0001 |
| Postoperative ECHO LAVVR grade, n (%) | 0.83 | ||
| None | 0 | 1 (4) | |
| Trivial | 3 (15) | 3 (12) | |
| Mild | 13 (65) | 16 (64) | |
| Moderate | 4 (20) | 5 (20) | |
| Severe | 0 | 0 | |
| 30-Day PPM insertion, n (%) | 1 (5) | 0 | 0.26 |
AVSD: atrioventricular septal defect; DOLAVV: double orifice left atrioventricular valve; DP: double patch; IQR: interquartile range; LAVVR ≥ 3: moderate or greater left atrioventricular valve regurgitation; LVOTO: left ventricular outflow tract obstruction; MSP: modified single patch; PAB: pulmonary artery banding; PPM: permanent pacemaker; SD: standard deviation.
Ventricular septal defect height
The VSD height in the MSP group ranged from 4.2 to 11.7 mm with a mean height of 7.3 ± 1.9 mm, and in the DP group, the VSD height ranged from 5.1 to 14.9 mm with a mean height of 8.3 ± 2.4 (Table 2). Figure 2 demonstrates the broad range of VSD heights at the time of CAVSD repair, with 9 patients (7 DP, 2 MSP) having a deep ‘scoop’, defined as a VSD height >10 mm. There was no significant difference in mean VSD height (P = 0.51), or deep ‘scoop’ (P = 0.13) between the 2 repair groups (P = 0.51, Table 2).
Table 2:
Echocardiographic features before complete atrioventricular septal defect repair
| Characteristics | MSP (n = 20) | DP (n = 25) | P-value |
|---|---|---|---|
| Mean VSD height, mean ± SD | 7.3 ± 1.9 | 8.3 ± 2.4 | 0.51 |
| Mean LV length, mean ± SD | 27.1 ± 4.4 | 28.6 ± 7.4 | 0.69 |
| Mean VSD height to LV length ratio, mean ± SD | 0.28 ± 0.08 | 0.30 ± 0.08 | 0.43 |
| Deep scoop (VSD height >10 mm), n (%) | 2 (10) | 7 (28) | 0.13 |
DP: double patch; LV: left ventricular; MSP: modified single patch; VSD: ventricular septal defect.
Figure 2:
(A) Graphical representation of VSD height and LV length in complete atrioventricular septal defect patients stratified by repair technique. (B) VSD height stratified by need for reoperation and complete atrioventricular septal defect repair type. LV: left ventricular; VSD: ventricular septal defect. Double patch (red diamond) and modified single patch (blue circle).
Ratio of ventricular septal defect height to left ventricular length
The ratio of the VSD height to LV length varied widely in both groups, ranging from 14% to 47% (Fig. 3A). There was no significant difference in the VSD height-to-LV length ratio between the 2 repair strategies (P = 0.43) or in LV length between groups (P = 0.69). Foreshortening was present in 7 patients (28%) in the DP group and 5 patients (25%) in the MSP group and was not significantly different between groups.
Figure 3:
(A) Graphical representation of VSD height-to-LV length ratio stratified by CAVSD repair technique. (B) VSD height in CAVSD patients stratified by Rastelli class and repair technique; CAVSD: complete atrioventricular septal defect; LV: left ventricular; VSD: ventricular septal defect. Double patch (red diamond) and modified single patch (blue circle).
Rastelli classification
Rastelli classification was confirmed at the time of surgery by the operator and included 20 Rastelli A, 1 Rastelli B and 24 Rastelli C patients. The distribution of Rastelli types did not differ significantly between the 2 repair groups (Table 1, P = 0.49). The height of the VSD did not correlate with a specific Rastelli class (Fig. 3B).
Reoperation and survival
Eight patients required reoperation during follow-up with 7 patients requiring reoperation during their index hospital admission. Indications for early reoperation were closure of residual VSD in 1 patient and significant LAVV regurgitation in 6 patients (Table 3). One patient who had undergone DP repair required late reoperation for LVOTO.
Table 3:
Reoperation after complete atrioventricular septal defect repair
| Characteristics | MSP (n = 20) | DP (n = 25) | P-value |
|---|---|---|---|
| 30-Day reoperation, n (%) | 4 (20) | 3 (12) | 0.46 |
| LAVV repair | 1 | 1 | |
| LAVV replacement | 2 | 0 | |
| Closure of residual VSD | 1 | 0 | |
| LAVV repair and closure of residual VSD | 0 | 2 |
DP: double patch; LAVV: left atrioventricular valve; MSP: modified single patch; VSD: ventricular septal defect.
Two patients with a deep VSD required reoperation. Both patients had undergone previous DP repair and required reoperation for LVOTO and severe LAVV regurgitation, respectively. Utilizing Cox regression, VSD height was not a significant predictor of reoperation (HR 0.95, 95% confidence interval 0.69–1.33; P = 0.77). There was no 30-day mortality. Two patients died during long-term follow-up due to cardiac failure and respiratory failure, respectively.
COMMENT
Repair of CAVSD is a challenging intra-cardiac operation and several factors influence the surgeon’s choice of repair technique including subtleties of the underlying anatomy, patient age and size, associated cardiac abnormalities, operative experience, and institutional repair preferences. Regardless of repair technique utilized, reoperation for LAVV dysfunction and LVOTO remain important long-term concerns. With evolution of the MSP technique, more focus has been placed on the extent and nature of the VSD with regards to repair technique and the risk of LAVV dysfunction and development of LVOTO post-CAVSD repair. Although there is no clear evidence that VSD height impacts outcomes following CAVSD repair, it is often considered as one of the criteria guiding choice of repair technique. Backer et al. [10] considered a VSD height of >12 mm to be an indication for DP repair. Importantly, our study suggests that a deep ventricular scoop is uncommon in CAVSD patients, with the VSD being ≤10 mm in 80% of patients in our cohort. Moreover, there does not appear to be a clear VSD height ‘cut-off’ at which risk the of reoperation increases.
Concern regarding the impact of a deep ventricular ‘scoop’ on the postoperative outcome after MSP repair of CAVSD continues to be an issue for some surgeons when considering whether to perform this technique. This relates to the distance that the AV valve leaflet tissue must be displaced down and fixed to the ventricular septal crest, which may alter LAVV function or result in further elongation of the LVOT when the VSD is deep. Although numbers are small and the overall differences were not significant, there appears to be a preference amongst surgeons in our unit towards repair with the DP technique when there is a deep VSD scoop defined by a VSD height >10 mm. Seven of these patients were in the DP group compared with only 2 in the MSP group; however, it is impossible to know whether the approach to these patients altered the overall outcome in this study. A larger study is required to address the question of whether the type of repair technique alters outcome in patients with a VSD height >10 mm.
Anatomical studies focusing on the morphological aspects of CAVSD illustrate the close relationship between the VSD height and LVOT diameter [15, 17]. A morphometric analysis by Adachi et al. [5] found 16 of 43 atrioventricular septal defect heart specimens had anterosuperior extension of the scoop, associated with a skewed shape of the scoop and significantly narrower LVOT. They suggested that not only the VSD height was important, but also the presence of anterosuperior extension could affect valvar competence after MSP repair. However, assessing for the presence of anterosuperior extension can be quite challenging with conventional 2D echocardiography techniques, and subsequent studies [1, 9] have failed to show a difference in reintervention for LVOTO between MSP and DP repair. With regard to LAVV function, there is evidence that the LAVV annulus has more systolic contraction with the MSP technique compared to DP repair with no other identifiable differences in mechanics of LAVV function following repair with the 2 techniques [14]. Despite the small cohort size, this finding suggests that LAVV function is not negatively impacted by use of the MSP technique as suggested by equivalent rates of LAVV reintervention in our study and previous studies [1].
2D echocardiography has been the cornerstone of CAVSD diagnosis and preoperative assessment [11], being both non-invasive and widely accessible. However, recent advances [12] in 3-dimensional echocardiography may provide the opportunity to better understand the anatomical defects of CAVSD due to better depth perception and resolution allowing better sensitivity in determining complex leaflet and LVOT abnormalities [18], as well as mechanisms for failed repair [19]. The issue with correlating preoperative CAVSD anatomy and long-term outcomes is the length of time it takes to accrue useful long-term follow-up data. Future studies correlating 3-dimensional echocardiography assessment and failed repair during long-term follow-up would be beneficial.
Limitations
This study was limited by its small sample size, retrospective nature, single-centre nature and the inability to control for surgeon preference or decision-making in choice of repair technique. All measurements were performed using available images stored from the patient’s preoperative transthoracic echocardiogram and were therefore limited to the imaging data available. The 2D definition of the transthoracic echocardiogram and any foreshortening may have resulted in underestimation of the LV length dimensions.
CONCLUSIONS
There was no correlation observed between the VSD height and risk of reoperation after CAVSD repair and mean VSD height was similar between repair techniques. Whilst a deep ventricular scoop is uncommon in CAVSD patients, there appears to be surgeon preference for the DP technique in these patients, and further studies on this particular subgroup are needed. Measurement of the VSD height should be an essential part of the standard preoperative assessment of CAVSD patients in the current era.
Funding
This study was supported by the National Heart Foundation of Australia [Health professional scholarship 101616 to L.S.F.]
Conflict of interest: none declared.
Author contributions
Laura S. Fong: Conceptualization; Data curation; Formal analysis; Writing—original draft. David Youssef: Data curation; Writing—review & editing. Julian Ayer: Supervision; Writing—review & editing. Ian A. Nicholson: Supervision. David S. Winlaw: Supervision; Writing—review & editing. Yishay Orr: Formal analysis; Methodology; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Amir-Reza Hosseinpour, Cheul Lee, Christoph Schmitz and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- 2d2
Dimensional
- CAVSD
Complete atrioventricular septal defect
- DP
Double patch
- HR
Hazard ratio
- LAVV
Left atrioventricular valve
- LV
Left ventricular
- LVOT
Left ventricular outflow tract
- LVOTO
Left ventricular outflow tract obstruction
- MSP
Modified single patch
- VSD
Ventricular septal defect
REFERENCES
- 1. Fong LS, Betts K, Bell D, Konstantinov IE, Nicholson IA, Winlaw DS. et al. Complete atrioventricular septal defect repair in Australia: results over 25 years. J Thorac Cardiovasc Surg 2019;30:30. [DOI] [PubMed] [Google Scholar]
- 2. Wilcox BR, Jones DE, Frantz EG, Brink LW, Henry GW, Mill MR. et al. Anatomically sound, simplified approach to repair of “complete” atrioventricular septal defect. Ann Thorac Surg 1997;64:487–93; discussion 493–4. [DOI] [PubMed] [Google Scholar]
- 3. Nunn GR. Atrioventricular canal: modified single patch technique. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2007;10:28–31. [DOI] [PubMed] [Google Scholar]
- 4. Nicholson IA, Nunn GR, Sholler GF, Hawker RE, Cooper SG, Lau KC.. Simplified single patch technique for the repair of atrioventricular septal defect. J Thorac Cardiovasc Surg 1999;118:642–6. [DOI] [PubMed] [Google Scholar]
- 5. Adachi I, Ho SY, McCarthy KP, Uemura H.. Ventricular scoop in atrioventricular septal defect: relevance to simplified single-patch method. Ann Thorac Surg 2009;87:198–203. [DOI] [PubMed] [Google Scholar]
- 6. Backer CL, Stewart RD, Bailliard F, Kelle AM, Webb CL, Mavroudis C.. Complete atrioventricular canal: comparison of modified single-patch technique with two-patch technique. Ann Thorac Surg 2007;84:2038–46; discussion 2038–46. [DOI] [PubMed] [Google Scholar]
- 7. Pan G, Song L, Zhou X, Zhao J.. Complete atrioventricular septal defect: comparison of modified single-patch technique with two-patch technique in infants. J Card Surg 2014;29:251–5. [DOI] [PubMed] [Google Scholar]
- 8. Yildirim O, Avsar M, Ozyuksel A, Akdemir M, Zeybek C, Demiroluk S. et al. Modified single versus double-patch technique for the repair of complete atrioventricular septal defect. J Card Surg 2015;30:595–600. [DOI] [PubMed] [Google Scholar]
- 9. Li D, Fan Q, Iwase T, Hirata Y, An Q.. Modified single-patch technique versus two-patch technique for the repair of complete atrioventricular septal defect: a meta-analysis. Pediatr Cardiol 2017;38:1456–64. [DOI] [PubMed] [Google Scholar]
- 10. Backer CL, Eltayeb O, Monge MC, Wurlitzer KC, Hack MA, Boles LH. et al. Modified single patch: are we still worried about subaortic stenosis? Ann Thorac Surg 2015;99:1671–5; discussion 1675–6. [DOI] [PubMed] [Google Scholar]
- 11. Santoro G, Marino B, Di Carlo D, Formigari R, Santoro G, Marcelletti C. et al. Patient selection for repair of complete atrioventricular canal guided by echocardiography. Eur J Cardiothorac Surg 1996;10:439–42. [DOI] [PubMed] [Google Scholar]
- 12. Colen TS, Jeffrey F.. Three-dimensional echocardiography for the assessment of atrioventricular valves in congenital heart disease: past, present and future. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2015;18:62–71. [DOI] [PubMed] [Google Scholar]
- 13. Al Senaidi KS, Ross DB, Rebeyka IM, Harder J, Kakadekar AP, Garros D. et al. Comparison of two surgical techniques for complete atrioventricular septal defect repair using two- and three-dimensional echocardiography. Pediatr Cardiol 2014;35:393–8. [DOI] [PubMed] [Google Scholar]
- 14. Ugaki S, Khoo NS, Ross DB, Rebeyka IM, Adatia I.. Modified single-patch compared with two-patch repair of complete atrioventricular septal defect. Ann Thorac Surg 2014;97:666–71. [DOI] [PubMed] [Google Scholar]
- 15. Penkoske PA, Neches WH, Anderson RH, Zuberbuhler JR.. Further observations on the morphology of atrioventricular septal defects.[Erratum appears in J Thorac Cardiovasc Surg 1988;95(1):146]. J Thorac Cardiovasc Surg 1985;90:611–22. [PubMed] [Google Scholar]
- 16. Fong LS, Betts K, Kannekanti R, Ayer J, Winlaw DS, Orr Y.. Modified-single patch vs double patch repair of complete atrioventricular septal defects. Semin Thorac Cardiovasc Surg 2019;12:12. [DOI] [PubMed] [Google Scholar]
- 17. Ebels T, Ho SY, Anderson RH, Meijboom EJ, Eijgelaar A.. The surgical anatomy of the left ventricular outflow tract in atrioventricular septal defect. Ann Thorac Surg 1986;41:483–8. [DOI] [PubMed] [Google Scholar]
- 18. Kutty S, Smallhorn JF.. Evaluation of atrioventricular septal defects by three-dimensional echocardiography: benefits of navigating the third dimension. J Am Soc Echocardiogr 2012;25:932–44. [DOI] [PubMed] [Google Scholar]
- 19. Takahashi K, Mackie AS, Thompson R, Al-Naami G, Inage A, Rebeyka IM. et al. Quantitative real-time three-dimensional echocardiography provides new insight into the mechanisms of mitral valve regurgitation post-repair of atrioventricular septal defect. J Am Soc Echocardiogr 2012;25:1231–44. [DOI] [PubMed] [Google Scholar]