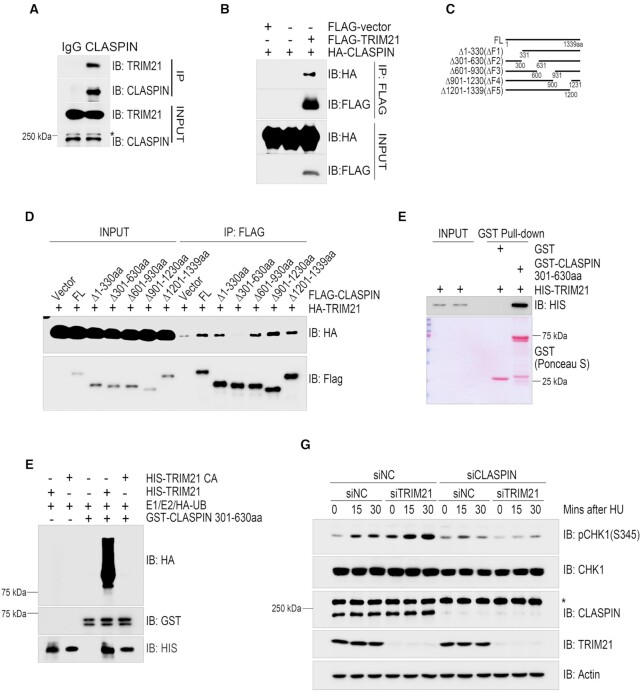
Figure 2.
TRIM21 ubiquitinated CLASPIN and regulated CLASPIN dependent CHK1 activation. (A) HEK293T cells were lysed and incubated with protein A agarose conjugated with normal rabbit IgG or anti-CLASPIN antibody for immunoprecipitation, followed by immunoblotting with the indicated antibodies. *, non-specific signal. (B) HEK293T cells co-transfected with HA-CLASPIN and FLAG-TRIM21 were lysed and incubated with anti-FLAG M2 agarose, the immunoprecipitates were then examined by immunoblotting with antibodies against HA and FLAG. (C) Schematic of the CLASPIN deletion mutants: F1:1–330aa, F2:301–630aa, F3:601–930aa, F4:901–1230aa, F5:1201–1339aa. (D) Whole cell lysates extracted from HEK293T cells co-transfected with HA-TRIM21 and wildtype or mutant FLAG-CLASPIN were incubated with anti-FLAG M2 agarose for immunoprecipitation, followed by immunoblotting with the indicated antibodies. (E) Bacterially-purified HIS-TRIM21 and GST-CLASPIN301-630aa/GST peptides immobilized on Glutathione Sepharose 4B beads were mixed and incubated for 4 h. The HIS-TRIM21 complex was examined by immunoblotting. GST-CLASPIN301-630aa/GST were directly visualized by Ponceau S staining. (F) In vitro ubiquitination assays were performed by incubating HIS-TRIM21 or its E3 ligase inactive mutant and GST-CLASPIN301-630aa in the presence of E1, E2 and HA-ubiquitin at 30°C for 1 h, followed by GST-pulldown and analysis by immunoblotting with the indicated antibodies. (G) HeLa cells were transfected with a negative control siRNA or an siRNA targeting TRIM21 or CLASPIN as indicated for 48 hours. Then, whole cell lysates were collected after being treated with 2 mM HU or a mock treatment for the indicated times. CHK1 activation was examined by immunoblotting with an antibody specifically recognizing phosphorylated CHK1 at Ser345. The expression levels of CLASPIN, TRIM21 and CHK1 were also examined. *Non-specific signal. Actin: loading control.