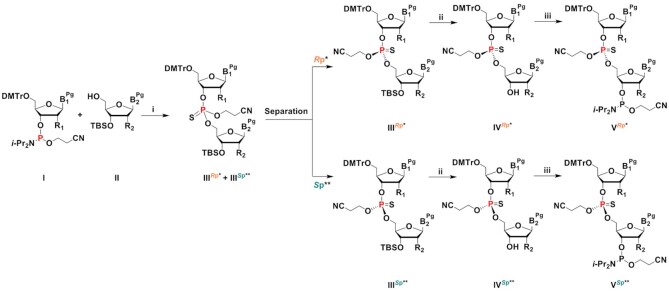
Scheme 1.
Synthesis and separation of chiral PS dinucleotides as building blocks for oligonucleotide synthesis. B = nucleobase, uracil (U), adenine (A), guanine (G); Pg = protecting group, benzoyl (Bz) for A, N-isobutyryl (i-Bu) for G; R1, R2 = F and/or OMe; DMTr = dimethoxytrityl; TBS = tert-butyldimethylsilyl; CE = β-cyanoethyl; * except dimer gsa (Sp); ** except dimer gsa (Rp) (i) (a) 5-ethylthio-1H-tetrazole (ETT), solvent, rt; (b) phenylacetyl disulfide (PADS), 2,6-lutidine, rt; (ii) Et3N·3HF, THF, rt to 50°C; (iii) 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite, 5-ethylthio-1H-tetrazole (ETT), solvent, 0°C to rt. Synthesis of each unique stereo-defined dinucleotide according to this scheme is described in detail in Materials and Methods and in Supplementary Information: IIIRp* refers to a, whereas the IIISp** refers to b of compounds 3, 9, 15, 21, 27, 33 and 39; IVRp* refers to a, whereas the IVSp** refers to b of compounds 4, 10, 16, 22, 28, 34, and 40; VRp* refers to a, whereas the VSp** refers to b compounds 5, 11, 17, 23, 29, 35 and 41.