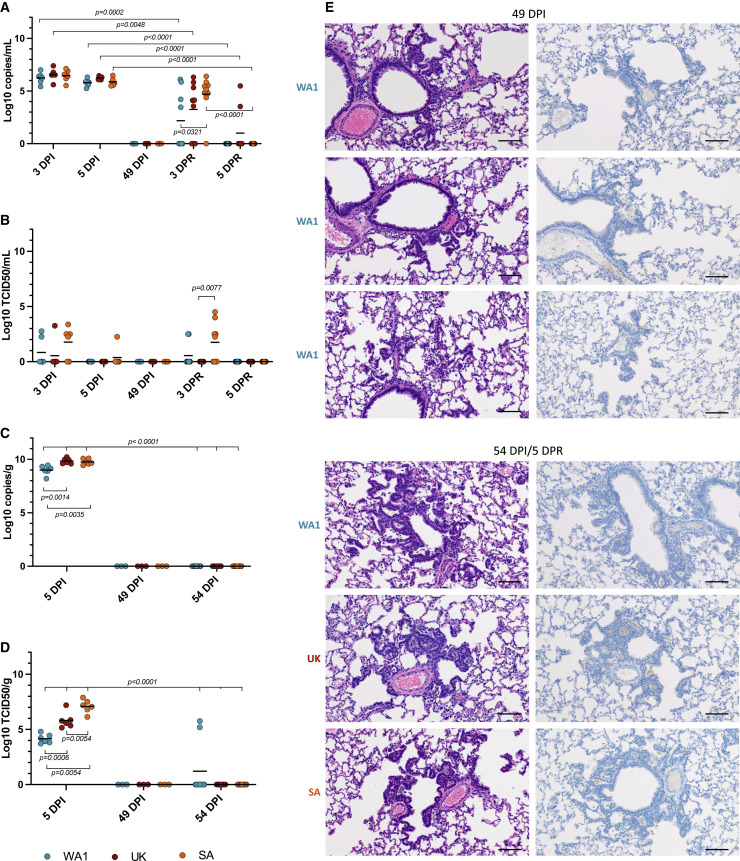
Figure 5.
Viral replication and pulmonary pathology after heterologous reinfection
A sgE qPCR assay was used to detect viral RNA loads in oral swabs and lung tissue (A, C). A standard TCID50 assay was used to determine levels of infectious virus in oral swabs and lung tissues (B, D). Hematoxylin and eosin (H&E) and immunohistochemistry (IHC) staining were used to assess histopathology and SARS-CoV-2 antigen distribution in lung tissue (E).
(A) Viral RNA in oral swabs.
(B) Infectious virus in oral swabs.
(C) Viral RNA levels in lung samples.
(D) Infectious virus in lung samples.
(E) Histology and SARS-CoV-2 antigen distribution in lung tissue. H&E (original magnification ×200) and IHC (SARS-CoV-2 N protein, original magnification ×200) staining were performed immediately before (49 DPI) and 5 days after heterologous reinfection (54 DPI/5DPR). H&E (left panels) showed resolving interstitial pneumonia developing into alveolar bronchiolization. Reinfection failed to induce histopathology consistent with acute SARS-CoV-2 infection (H&E, 200 × 54 DPI/5 DPR. IHC (right panels) showed that resolving interstitial pneumonia and alveolar bronchiolization were not associated with SARS-CoV-2 immunoreactivity. Reinfection failed to result in SARS-CoV-2 specific immunoreactivity (IHC, original magnification ×200 at 54 DPI/5 DPR), (scale bar 100 μM). A nonparametric one-way AVOVA (Kruskal-Wallis) with correction for multiple comparisons was used to determine statistical differences in PRISM. WA1 reinfection (blue), B.1.1.7 reinfection (red), and B.1.351 reinfection (orange).