Figure 3.
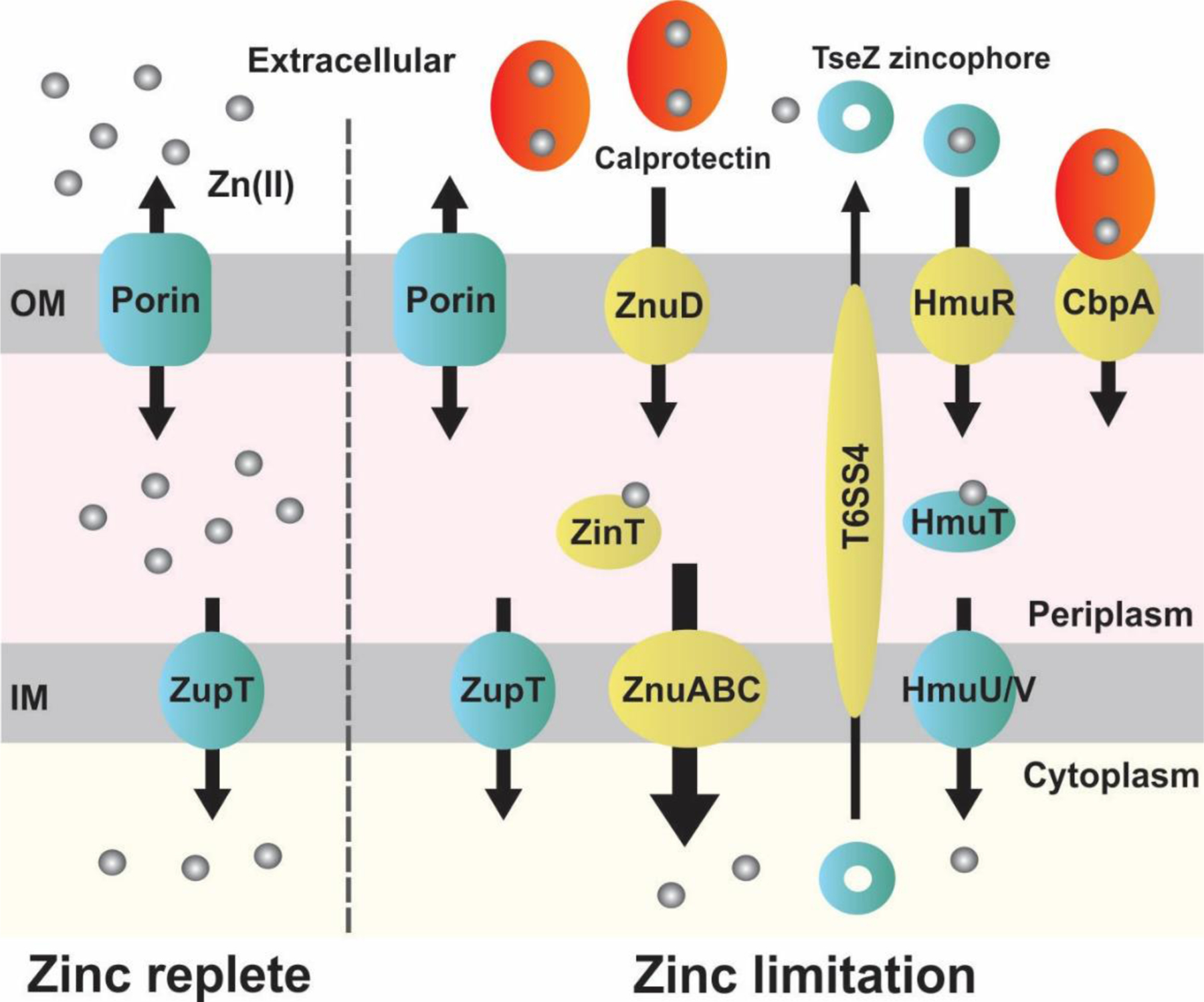
Mechanisms of Zn(II) internalization in Gram-negative bacteria. In Zn(II)-replete conditions (left), Zn(II) ions enter the periplasmic space through non-specific porins and are transported to the cytoplasm by constitutively expressed antiporters, such as ZupT (in E. coli) [56]. Upon Zn(II) limitation (right), the Zur regulon is de-repressed and additional importers and accessory proteins are produced (highlighted in yellow). These include the high-affinity importer ZnuABC [10], widely-conserved in bacteria, the periplasmic zinc-binding protein ZinT from Enterobacterales [57], which delivers Zn(II) ions to ZnuABC, the TonB-dependent ZnuD importers from Neisseria meningitidis and non-fermenters [10], the capture of extracellular Zn(II) through secretion and incorporation of zincophores, such as TseZ from Burkholderia thailandensis [58], and receptors specific for Zn(II)-bound calprotectin, such as CbpA from N. meningitidis [59] or TdfH from Neisseria gonorrhoeae [60], that strip Zn(II) from it. IM: inner membrane; OM: outer membrane.
