Figure 1. IgG glycosylation and glycoengineering of IgG antibodies using ENGases and glycosynthase mutants.
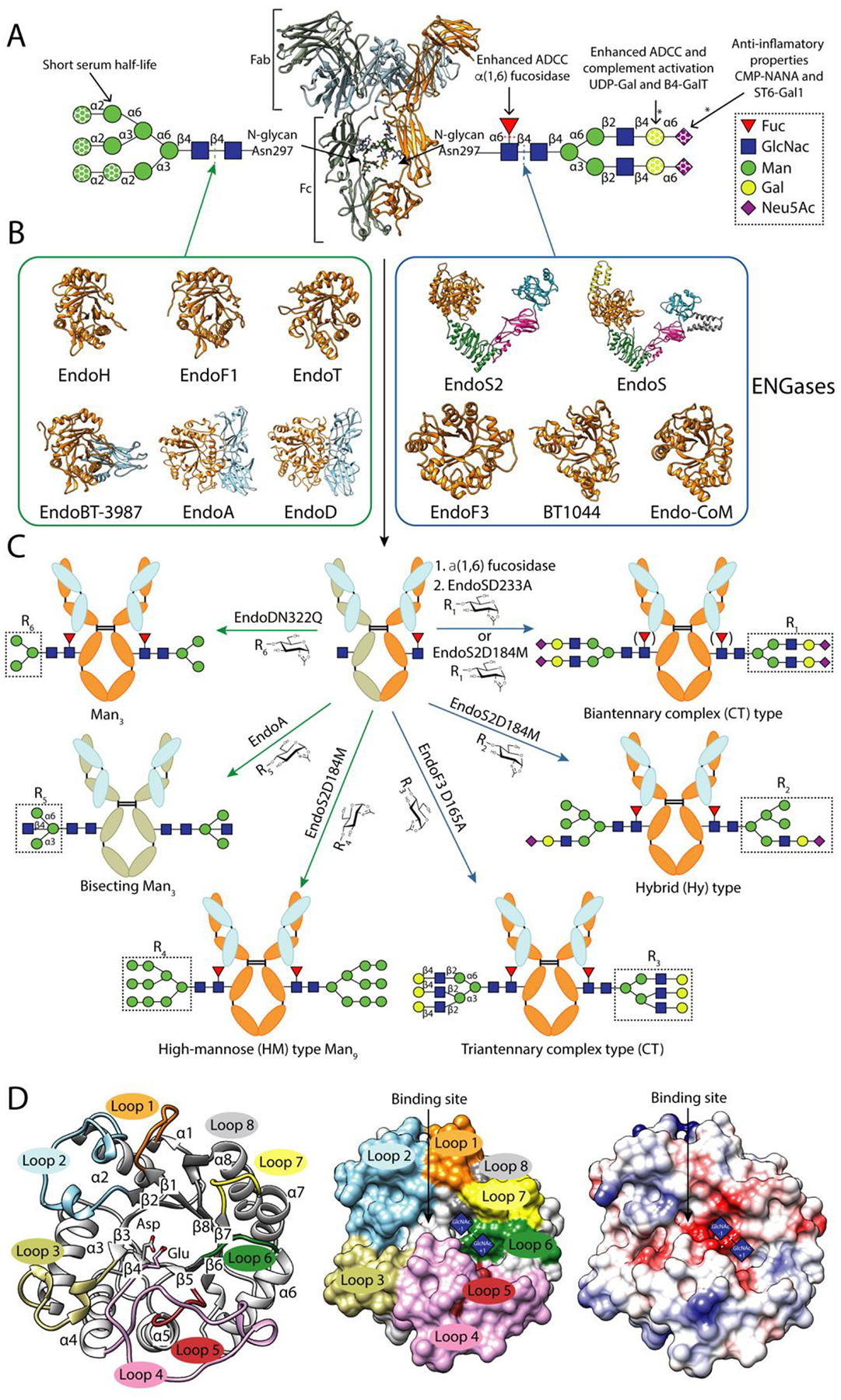
A. Cartoon representation of the overall structure of a human IgG antibody (PDB code 1HZH). Human IgG antibodies are composed of two identical heavy chains (HC) and two identical light chains (LC) organized in three globular domain structures: two identical antigen-binding fragments (Fab) and the crystallizable fragment (Fc) region. Main N-glycans found at Asn297 of Fc region in recombinant and biological IgG antibodies. In addition, 15–25% of the human serum IgG antibodies carry a second N-glycan in the LC of the Fab region [65]. This N-glycan is also highly variable, comprising over 37 different glycoforms, affecting the immunoreactivity, antigen affinity and half-life of the antibody [66]. All eukaryotic N-glycans share a common core sequence, Manα1–3(Manα1–6)Manβ1–4GlcNAcβ1–4GlcNAcβ1–Asn-X-Ser/Thr, and are classified into three major types according to the composition and structural arrangement of the α(1,3) and α(1,6) antennae: complex-type (CT-type), high-mannose-type (HM-type), and hybrid type (Hy-type) [67]. The structural repertoire of N-glycans is further expanded by (i) additional antennae (e.g., bisecting, tri-antennary and tetra-antennary CT-type N-glycans), (ii) the addition of sugars to the N-glycan core (e.g., α(1,6)-Fuc to the Asn-linked GlcNAc), and (iii) the elongation of antennae (e.g., the addition of α(2,6)-Neu5Ac to the terminal Gal in CT-type N-glycans)IgGs from human serum are composed of up to 33 different glycoforms, the most predominant N-glycan being the fucosylated biantennary CT-type [68]. Fc N-glycan structural heterogeneity markedly impacts the functionality, immunogenicity and pharmacokinetics of the IgG antibodies [69]. The dotted shapes represent that carbohydrate may or may not be present, indicating the high structural variability of the N-glycan. The lack of α(1,6)-Fuc increases the binding of IgGs to FcγRIIIa, enhancing the antibody-dependent cellular cytotoxicity (ADCC) [16,17], and in some cases improving their antitumoral activity. The addition of terminal α(2,6)-Neu5Ac to the Fc region of IgGs is associated with increased anti-inflammatory properties [70]. The presence of Gal residues is important for IgGs to interact with C1q and activate the complement pathway [71]. IgG antibodies bearing HM-type N-glycans have a shorter half-life in humans than antibodies with other glycoforms, increasing serum clearance [72].The reactions catalysed by glycosyl hydrolyses and glycosyltransferases in order to modify the N-glycan composition are marked by dotted lines and asterisks, respectively. B. Characterized ENGases from the GH18 and GH85 family with known crystal structure classified in two groups according to the N-glycans they can hydrolyze: HM-type N-glycans (green square) and CT-type N-glycans (blue square). C. In vitro chemoenzymatic remodeling of IgG antibodies. After hydrolysis of heterogenous IgG antibody glycoforms by ENGases, glycosynthase mutants can transfer a defined glycan from an oxazoline donor to the remaining GlcNAc attached to Asn297, according to the enzyme N-glycan specificity. In addition, AlfC, an α-fucosidase that only hydrolyze fucose from single GlcNAcs attached to Asn297, can first be used to remove this carbohydrate moiety that reduces the binding to FcγRIIIa. The HCs of the IgG substrates bear HM-type N-glycans (grey) or CT-type N-glycans (orange). The LC is highlighted in light blue. D. Cartoon (left), surface (center) and coulombic surface (right) representations of the X-ray crystal structure of EndoF3 in its unliganded (PDB code 1EOK), showing the overall structure of the glycosyl hydrolase domain of an ENGase. The β-strands and the α-helices that formed the (β/α)8 TIM-barrel and the loops surrounding the binding site that defined the N-glycan specificity of this enzymes are annotated. Members of both families follow a conserved substrate-assisted mechanism with retention of the anomeric configuration. In a first step, a glutamic acid acts as an acid and protonates the anomeric carbon and an aspartic acid (GH18 family) or asparagine (GH85 family) stabilizes the reaction intermediate and orients the oxygen of the 2-acetamide groupo of GlcNAc (−1) that attacks the anomeric carbon and forms an oxazolinium intermediate. In a second step, the glutamic acid acts as a base and deprotonates a water molecular provoking the second nucleophilic attack and the hydrolysis of the oxazolinium intermediate. The catalytic residues, aspartic and glutamic acids, are highlighted and the sugar binding subsites of GlcNAcs (+1) and (−1) are represented by blue squares.
