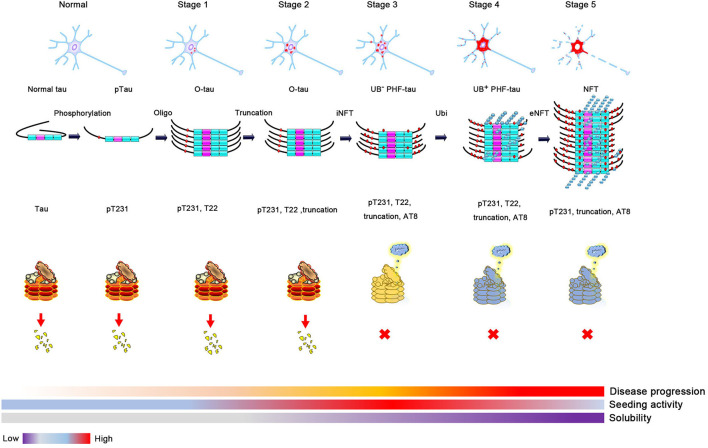
Figure 2.
The proposed process of tau aggregation. Phosphorylation changes tau conformation and induces oligomer tau aggregation. Soluble oligomer tau has higher toxicity and seeding activity. Proteases, such as caspase 3, calpain, or legumain, can cleavage tau and result in tau truncation when the cell is toxic. Monomer and oligomer tau can be ubiquitinated and degraded by the proteasome. However, ubiquitin-positive tau aggregation will be accumulated when the activity of proteasome is decreased (high activity, red; medial activity, yellow; and low activity, blue), which will decrease the seeding activity and solubility of tau aggregation and finally lead to the formation of neurofibrillary tangles formation.