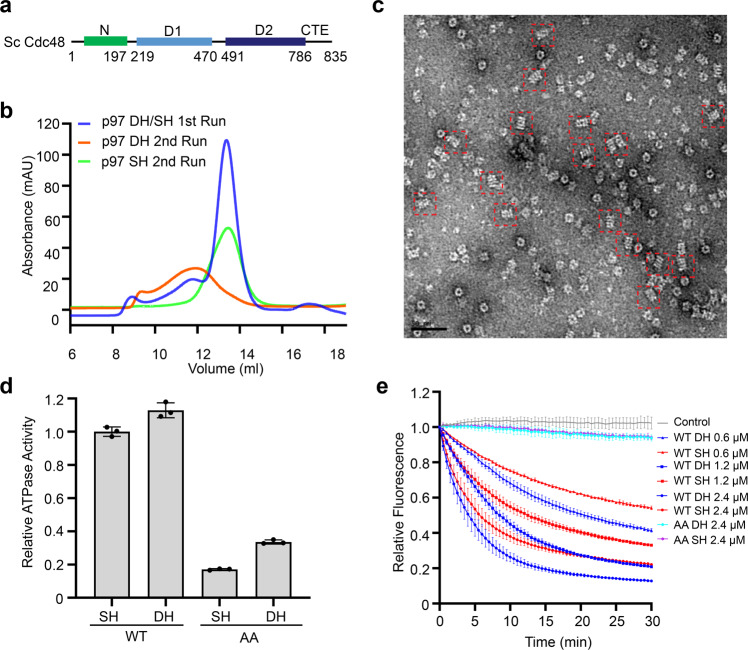
Fig. 2. p97/Cdc48 double hexamers (DHs) are active ATPases.
a Domains and motifs of Saccharomyces cerevisiae (Sc) Cdc48. b Gel filtration profiles of human p97 on a Superose 6 column. c Representative negative stain EM micrograph of human p97 DH. DHs in side views are boxed by red dashed squares. d Relative ATPase activities of the indicated human p97 DHs and single hexamers (SHs) at the protomer concentration of 120 nM. WT, wild type; AA, E305A/E578A, p97 mutant deficient in ATPase activities of both D1 and D2 domains. The activities were normalized to that of WT SH. Data are shown as means ± SEM (n = 3 independent experiments). e Substrate unfolding assay by the indicated Sc Cdc48 DHs and SHs at varying protomer concentrations. WT, wild type; AA, E315A/E588A, Cdc48 mutant deficient in ATPase activities of both D1 and D2 domains. Data are shown as means ± SEM (n = 3 independent experiments).