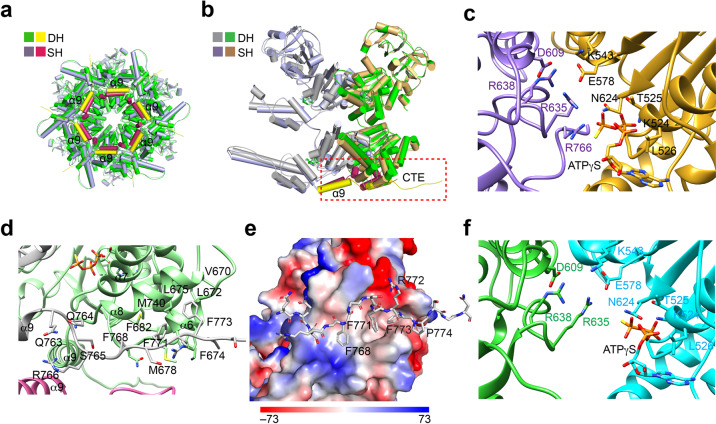
Fig. 4. The C-terminal extension (CTE) tethers the D2 ring in p97 double hexamer (DH).
a Superimposition of ATPγS-bound SH conformer I and the hexamer in DH conformer II. The ɑ9 helices of SH and DH are colored yellow and red, respectively. Other structural elements of SH and DH are colored light blue and green. b Superimposition of two adjacent protomers from SH and DH. The two protomers from DH are colored gray and green, while the SH protomers are colored light blue and gold. ɑ9 and CTE are boxed with red dashed lines. c The ATP-binding pocket of the D2 ring in SH conformer I. d Interactions involving the CTE in the DH. e Surface diagram of the CTE-binding site colored by electrostatic potential (red, negative; blue, positive; white, neutral). The CTE is shown as sticks. f The ATP-binding pocket of the D2 ring in DH conformer II.