Abstract
Thrombosis that occurs in coronavirus disease 19 (COVID-19) is a serious complication and a critical aspect of pathogenesis in the disease progression. Although thrombocytopenia is uncommon in the initial presentation, it may also reflect disease severity due to the ability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to activate platelets. This occurs directly through the spike protein-angiotensin converting enzyme 2 (ACE2) interaction and indirectly by coagulation and inflammation activation. Dysregulation in both innate and adaptive immune systems is another critical factor that causes thrombosis and thrombocytopenia in COVID-19.
Vaccination is the most potent and effective tool to mitigate COVID-19; however, rare side effects, namely vaccine-induced immune thrombotic thrombocytopenia (VITT)/thrombosis with thrombocytopenia syndrome (TTS) can occur following adenovirus-vectored vaccine administration. VITT/TTS is rare, and thrombocytopenia can be the clue to detect this serious complication. It is important to consider that thrombocytopenia and/or thromboembolism are not events limited to post-vaccination with vectored vaccine, but are also seen rarely after vaccination with other vaccines.
Various conditions mimic VITT/TTS, and it is vital to achieving the correct diagnosis at an earlier stage. Antiplatelet factor 4 (PF4) antibody detection by the enzyme-linked immunosorbent assay (ELISA) is used for diagnosing VITT/TTS. However, false-positive rates also occur in vaccinated people, who do not show any thrombosis or thrombocytopenia. Vaccinated people with messenger RNA vaccine can show positive but low density and non-functional in terms of platelet aggregation, it is vital to check the optical density. If anti-PF4 ELISA is not available, discriminating other conditions such as antiphospholipid syndrome, thrombotic thrombocytopenic purpura, immune thrombocytopenic purpura, systemic lupus erythematosus, and hemophagocytic syndrome/hemophagocytic lymphohistiocytosis is critical when the patients show thrombosis with thrombocytopenia after COVID-19 vaccination.
Keywords: COVID-19, Vaccine, Thrombosis, Thrombocytopenia, Coagulopathy
Introduction
Coronavirus disease 2019 (COVID-19) is highly thrombogenic, reflecting multiple thromboinflammatory pathways, including cellular, tissue, and endothelial injury in the pathogenesis of COVID-19 [1]. Distinct from the thrombogenicity in COVID-19, a peculiar thrombotic and thrombocytopenic complication can occur after vaccination with adenovirus-vectored vaccines, known alternatively as vaccine-induced immune thrombotic thrombocytopenia (VITT) or thrombosis with thrombocytopenia syndrome (TTS) [2,3]. Although the incidence is low, thrombotic events with or without thrombocytopenia can occur after vaccination with all vaccines, and they are easily misdiagnosed as TTS/VITT [4]. These post-vaccination thromboses/thrombocytopenia mechanisms are not fully elucidated; however, the presence of common immune derangements as recognized in COVID-19-associated coagulopathy is suspected [5]. One year has passed since the COVID-19 vaccine programs were initiated, and cases of post-vaccine thrombosis have been reported with the increasing numbers of vaccinations. Since the number of potential thrombosis/thrombocytopenia cases will inevitably increase along with the growing number of vaccine recipients, we summarize the current knowledge regarding the thrombotic and/or thrombocytopenic disorders reported with the COVID-19 vaccinations.
Thrombosis
COVID-19
According to a US registry, the incidence of thrombotic complications in patients with COVID-19 is high: 2.6% in non-critically ill hospitalized patients and 35.3% in critically ill patients [6]. The pathophysiology of thrombosis is complex, but pneumocytes infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) trigger local inflammation, tissue damage, and microvascular thrombosis within the lung [7]. Subsequently, inflammation, coagulopathy, and endothelial damage expand systemically in severe cases. Such a pathway resembles that recognized in sepsis-induced coagulopathy, which can progress to overt disseminated intravascular coagulation and thrombosis [8].
In addition to the mechanisms mentioned above, COVID-19 specific pathways of coagulation activation can also occur. SARS-CoV-2 infects host cells through the binding of spike protein to angiotensin-converting enzyme 2 (ACE2) receptors which are expressed on monocytes, macrophages, platelets, and endothelial cells. In endothelial cells and platelets, spike protein-ACE2 binding shifts their function toward procoagulant and thrombogenic [9]. Zheng et al. [10] found that the spike protein can competitively inhibit binding of antithrombin and heparin cofactor II to heparan sulfate of the endothelial glycocalyx, causing increased thrombogenicity, mechanisms that further contribute to COVID-19-associated coagulopathy. However, spike protein-induced coagulopathy may also provide a potential explanation also for rare episodes of thrombosis reported post-vaccination.
In COVID-19 injury, the lung microvasculature is an initial site of thrombus formation, but thrombosis can occur extrapulmonary as well. Thromboses in cerebral venous sinuses, and splanchnic veins (portal, mesenteric) are the hallmarks of VITT/TTS, but the incidence of these unusual thromboses is also increased in COVID-19. Taquet et al. [11] reported the incidences of cerebral venous sinus thrombosis (CVST) and portal vein thrombosis two weeks following COVID-19 diagnosis to be 42.8 per million people (95% confidence interval [CI], 28.5–64.2) and 392.3 per million people (95% CI, 342.8–448.9), respectively, and those incidences were higher than those in vaccinated non-COVID matched cohorts. Because of immunopathogenic mechanisms of COVID-19 injury, there are significant similarities to those in autoimmune diseases, and as a result, it is not surprising that COVID-19-associated coagulopathy can present similarly to other autoimmune thrombotic diseases, such as antiphospholipid syndrome (APS), thrombotic thrombocytopenic purpura (TTP), and systemic lupus erythematosus (SLE) [12].
Virus-vectored vaccine
Within six months after the initiation of vaccination, extremely rare but unique thromboembolic events with thrombocytopenia that typically present in CVST and splanchnic vein thromboses were reported [2,3]. Although the pathophysiology has not been fully elucidated, high levels of platelet-activating antiplatelet factor 4 (PF4)/polyanion antibodies have been implicated [13]. Ordinary (vaccine-unrelated) thrombosis in cerebral veins typically develops independently of anti-PF4/polyanion antibodies. Sánchez van Kammen et al. [14] examined the blood samples in 952 CVST obtained pre-COVID-19 era and reported 8.4% had mild thrombocytopenia, and only one patient was positive for anti-PF4/heparin antibodies. Thus, the mechanism of vaccine-unrelated CVST may differ from that of VITT/TTS. In another study, 213 post-vaccination CVST cases (ChAdOx1 [Oxford/AstraZeneca]: 187 cases, BNT162b2 [Pfizer-BioNTech]: 25 cases, messenger RNA-1273 (mRNA) [Moderna]: 1 case) were reported to the European Medicines Agency is analyzed. Thrombocytopenia was reported in 107 cases (57%) in ChAdOx1 group, and none showed thrombocytopenia in the mRNA vaccine group. Ad26.COV2 vaccine
Although the anti-PF4 antibody was not examined, VITT/TTS is also recognized after Ad26.COV2 (Johnson & Johnson/Janssen) vaccination and suspected to occur only after the vaccination with adenovirus-vectored vaccines [15,16].
The mechanism of VITT/TTS resembles that of heparin-induced thrombocytopenia (HIT), as both reflect a pathogenic role for platelet-activating anti-PF4 antibodies. The causative IgG anti-PF4/polyanion antibody induces platelet activation and aggregation by cross-linking Fcγ receptor IIA on platelets and leads to thrombosis [17]. Interestingly, this antibody binds to the heparin-binding site on PF4, whereas HIT antibodies typically bind to heparin-dependent antigens elsewhere on PF4 [18]. In the case of VITT/TTS, the immune-triggering source of polyanion is still uncertain, but DNA from the vector adenovirus, other vaccine components, including hexon protein, and DNA from neutrophil extracellular traps are the possible candidates [19,20]. Even those are the cases, the mystery is the lack of similar VITT/TTS-like events in the people who received other adenovirus-vectored vaccines such as Ebola and AIDS vaccines [21]. If the vector-adenovirus cannot explain the whole story, other missing factors should be involved in the development of VITT/TTS. One possibility is that the spike protein plays a key role in developing thrombosis [5]. Since platelets express high levels of ACE2 [22], and the spike protein stimulates the release of PF4 from platelets, this spike protein-induced PF4 release can be the connecting link of VITT/TTS pathogenesis (Fig. 1 ). On the other hand, the mechanism cannot be explained by the cross-reactivity between spike protein and PF4 because anti-PF4 antibody does not react with the spike protein [23].
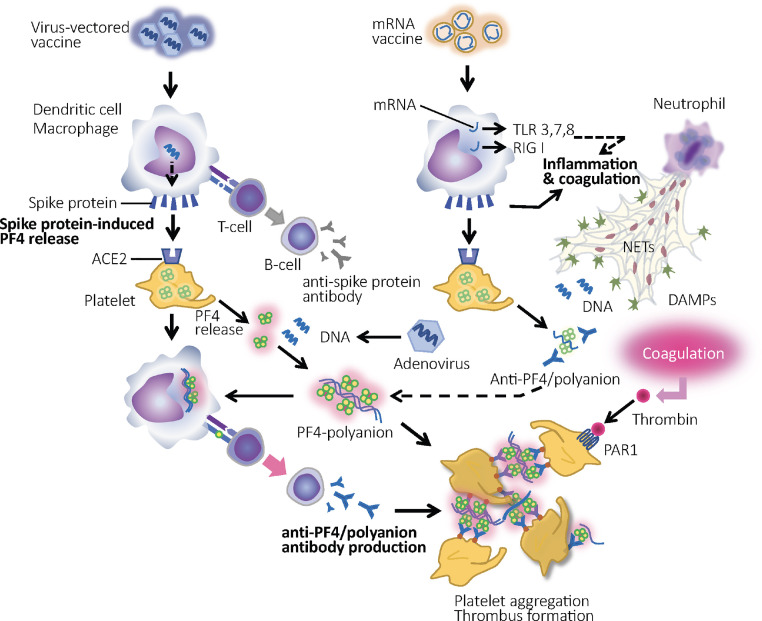
Fig. 1.
Mechanisms of thrombosis and thrombocytopenia after vaccination against COVID-19. Both adenovirus-vectored vaccine and messenger RNA (mRNA) vaccine induce spike protein production, which leads to the platelet factor 4 (PF4) release from platelets. In the case of vectored vaccine, DNA or other substances of the adenovirus binds to the PF4 as polyanion and form PF4/polyanion complex. After the conformational changes, PF4 expresses antigenicity, and the production of anti-PF4/polyanion antibody that leads to vaccine-induced immune thrombotic thrombocytopenia (VITT)/thrombosis with thrombocytopenia syndrome (TTS) is introduced. However, this reaction is unlikely to occur after mRNA vaccine administration because of the absence of polyanions. To provide antigenic PF4, polyanions such as DNA from the vector adenovirus and hexon protein in vectored vaccine are needed. Basically, ordinary RNA induces both inflammation and coagulation through the recognition by toll-like receptor 3, 7, 8, and retinoic acid-inducible gene I protein (RIG-I) and subsequent proinflammatory cytokine production. Although these unfavorable reactions are modulated significantly in mRNA vaccines, complete abolishment cannot be guaranteed. In addition, inflammation-induced thrombin, neutrophil extracellular traps (NETs), and damage-associated molecular patterns (DAMPs) potentially elicit platelet activation. PAR-1: protease-activated receptor 1.
Do thromboembolic events increase with vaccination? Hippisley-Cox et al. [24] examined the association between COVID-19 vaccines and the risk of thrombosis. According to the UK data, which accumulated over 29,000,000 people vaccinated with either ChAdOx1 (virus vector) or BNT162b2 (mRNA) and over 1700,000 people with positive SARS-CoV-2 testing, the risk ratio (compared exposed with unexposed periods in the same patient) of venous thromboembolism after ChAdOx1 vaccination and BNT162b2 vaccination seems to stay low: 1.10 (95% CI, 1.02–1.18 at 8–14 days) and 0.99 (95% CI, 0.90–1.08). Whereas, the risk ratio was 13.86 (95% CI, 12.76–15.05) after SARS-CoV-2 infection. Meanwhile, the risk of CVST was higher after ChAdOx1 vaccination and COVID-19 with risk ratios of 4.01 (95% CI, 2.08–7.71 at 8–14 days) and 13.43 (95% CI, 1.99–90.59). On the contrary, the risk was 2.57 (95% CI, 0.85–7.78 at 15–21 days), and the increase was not statistically significant after BNT162b2 vaccination. Therefore, it seems thrombotic complications, including CVST increased after ChAdOx1 vaccination, but the risk is much higher in COVID-19. Meanwhile, the risk may not increase over background following vaccination with BNT162b2.
Other vaccines
The proinflammatory response triggered by viral illness is a potential risk factor for thromboembolic complications. Vickers et al. [25] reported on venous thromboembolism (VTE) in adults over 50 years of age who received influenza vaccine (with or without pandemic H1N1) from 2007 through 2012. Cases were validated by medical record review, and the incidence and rate ratio were calculated for 1–10 days after vaccination relative to other person-times. From 1488 presumptive cases, 508 were reviewed, and 492 (97%) were confirmed cases of VTE. The authors reported no increased risk of VTE in the 1–10 days after influenza vaccination (Incidence Rate Ratio = 0.89, 95% CI, 0.69–1.17) compared to the control period. Results were similar when all person-time was censored before vaccination. No clustering of VTE was observed in the 1–42 days after vaccination.
As noted, Hippisley-Cox et al. [24] have shown that the rates of thromboembolism and CVST did not increase significantly after vaccination with BNT162b2. By contrast, Burn et al. [26] compared the rates of thromboembolism following vaccination with BNT162b2 in over 900,000 recipients, and reported a ratio of 1.29 [95% CI, 1.13–1.48] after the first vaccination. Burn et al., performed a similar survey in the different populations and reported the incidence ratio for pulmonary embolism was 1.21 (95% CI, 1.07–1.36) after vaccination with BNT162b2, which was consistent with that seen after ChAdOx1 vaccination [27]. Again, it should be reminded the increased risk was much higher in COVID-19 patients with an incidence of 15.31 (95% CI, 14.08–16.65). It is noteworthy that although the incidence of thromboembolism increased in their reports, the incidence of thromboembolism with thrombocytopenia did not increase after BNT162b2 vaccination. In another study, Barda et al. [28] reported neither deep vein thrombosis nor pulmonary embolism increased in over 880,000 vaccinated people with BNT162b2 in Israel. Furthermore, additional reports from an interim analysis of safety surveillance data from Vaccine Safety Datalink of over 6.2 million individuals in the US did not show a statistically significant increase in thrombotic events after either BNT162b2 or mRNA-1273 vaccines [4]. Whether or not the frequency of thrombosis increases depends on the study, but the epidemiological survey cannot rule out the thrombotic pathogenicity. Above survey reported the increased incidence of TTP and the risk ratio compared on the same calendar day was reportedly 2.60 (95% CI, 0.47–20.66). In summary, the incidence of thrombosis increases after vaccination with any vaccines including mRNA vaccines is minimal, but the association for other causes of thrombotic events should be carefully examined as part of any vaccine studies and evaluation.
Hypothetical background of thrombosis
In the case of virus-vectored vaccine, it is evident that anti-PF4/polyanion antibody is directly involved in the pathogenesis of thrombosis. However, evaluating anti-PF4/polyanion antibodies post-vaccination is a novel evaluation in recent years that can likely be seen with all vaccinations. Thiele et al. [29] reported the positive rate of anti-PF4/polyanion antibody was 8.0% in the vaccinated people with ChAdOx1, and that was 5.5% in the vaccinated people with BNT162b2. However, the optical density was low and less than 1.0 in most cases, and none were complicated with thrombosis in their study. The presence of anti-PF4 antibody is also recognized in COVID-19 patients. Greinacher et al. [23] reported 8.6% (19 of 222) patients were positive for anti-PF4 antibody, while only 2.7% of the total expressed high levels of antibodies (optical density > 1.0). Although the presence of anti-PF4 antibody does not directly induce thrombosis, it may reflect the increased PF4, which can be involved in the thrombogenesis. Other than platelet activation, the physiological roles of PF4 are neutralizing antithrombogenicity of the vascular endothelium by binding to heparan sulfate [30,31], and the neutralizing effect of PF4 on heparin has been shown [32]. PF4 also helps the bacteriocidal effect by binding to bacteria polyanions, thereby contributing to the host defense [33]. As a result, COVID-19 vaccination elicits an inflammatory response similar to the condition seen in sepsis, reducing the vascular anti-thrombogenicity potentially as a host response that developed to prevent the pathogen dissemination.
If mRNA COVID-19 vaccine can cause thrombosis, one possible explanation is a general procoagulant and proinflammatory effects associated with the immune responses to nucleic acid. With years of extensive research, this associated effect has been significantly improved in mRNA vaccines. For mRNA vaccines, nucleic acid is recognized by pattern-recognition receptors such as toll-like receptors and retinoic acid-inducible gene I protein (RIG-I), and potentially induces inflammatory reactions, but modification of mRNA by replacing uridine with pseudouridine attenuated immune activation [34]. However, the possibility of some residual inflammatory reactions affecting the thrombogenicity cannot be completely excluded. Another explanation is the spike protein-induced inflammation which is similar to that seen in vectored vaccine. After vaccination, synthesized spike protein is usually processed for antibody production and degraded by antigen-presenting cells. However, it cannot be excluded that in some cases, synthesized spike proteins are released into the circulation to produce unexpected immune responses via the binding to ACE2 [35]. Finally, the primary role of all vaccines is the modulation of immune systems, of course, and the activated autoimmune reaction can cause inflammation and induce procoagulant change, as seen in COVID-19. Again, for all of these critically important vaccines, the benefits far exceed the risks.
Thrombocytopenia
COVID-19
Thrombocytopenia can occur as a result of coagulation activation, subsequent inflammation, and systemic endothelial damage. Consequently, thrombocytopenia is recognized as a prognostic marker for COVID-19 [36]. In severe cases, the hyperactivation of the innate immune system that increases cytokines can induce a syndrome that mimics hemophagocytic syndrome (HPS)/hemophagocytic lymphohistiocytosis (HLH) and leads to thrombocytopenia [37]. Besides, platelet-activating immune complex and spike protein has been reported to stimulate platelets to release procoagulant factors such as PF4 and von Willebrand factor, and promote the aggregation of platelets [38]. The mechanism of how the spike protein stimulates platelets is not fully clarified, but since PF4 is essentially anti-pathogenic and the level is significantly elevated in sepsis as well as COVID-19 [39,40], it is natural to think that thrombocytopenia is induced by increased PF4-mediated immunothrombus formation [5]. If this is the case, PF4-induced thrombocytopenia is understood as a part of the natural host defense against COVID-19.
The cases should be much smaller, but since heparins are commonly used to treat COVID-19, HIT in COVID-19 may also be a part of the COVID-19-associated thrombocytopenia. Delrue et al. [41] examined the anti-PF4 antibody in adult COVID-19 patients and reported 11% of positive anti-IgG antibodies (optical density > 0.5), and the prevalence of HIT was 0.16%. This result also reminds us that despite the high positive rate of anti-PF4 antibodies, the titration is usually not high enough to induce platelet aggregation, and the prevalence of HIT is similar to the incidence reported previously in non-COVID-19 critically ill patients (0.20 to 0.45%) [42]. Patell et al. [43] also examined the HIT antibody in COVID-19 patients by latex aggregation test and reported that the cumulative incidence of positive HIT assay was 12% at 25 days (95% CI, 4%–26%). Thus, it should be kept in mind that among the COVID-19 patients, both ELISA and the rapid tests can be positive regardless of their relevance to thrombocytopenia.
Virus-vectored vaccine
The hematological feature of HIT is thrombocytopenia. Thrombosis is a major threat, but it is not always seen in HIT (50–70% of HIT patients develop thrombosis [44]), and thrombocytopenia accompanied by thrombosis is sometimes called heparin-induced thrombocytopenia with thrombosis (HITT) as a more specific term. Similarly, although thrombosis is believed to complicate most suspected VITT/TTS cases, it may not always present with thrombocytopenia. In addition, CVST and portal vein thrombosis are not easily detected initially, and the early stage VITT/TTS can easily be overlooked. Salih et al. [45] presented the cases with vaccine-induced thrombocytopenia (VIT) without associated CVST or other thromboses and with a severe headache. They cautioned that headache and thrombocytopenia are the preceding signs of CVST and should be captured as “pre- VITT syndrome.” Therefore, platelet count and D-dimer should be checked when patients complain of headache 5 to 28 days after vaccination with virus-vectored vaccines.
The critical question is how the thrombocytopenia occurs after vaccination. As introduced previously, Azzarone et al. [35] described the possibility of the synthesized spike protein-induced platelet activation and thrombocytopenia. In that theory, neosynthesized spike protein acts on inflammatory cells and platelets to damage endothelial cells in some vaccinated individuals with a predisposing environment. Since thrombocytopenia is recognized in very limited people, some unidentified conditions will be involved.
Separate from the story regarding VIT, Kuter [46] reported a transient decrease in platelet count in vaccinated chronic ITP patients at a significantly higher rate than expected. Moreover, vaccine‐induced thrombocytopenia is associated with many types of adenovirus-vectored vaccines, and triggering vaccine-induced ITP could potentially occur as an autoimmune mechanism may also contribute to vaccine-induced thrombocytopenia. The definitive diagnosis of ITP is usually made by ruling out other diseases. The platelet-associated IgG is not often measured because of the low sensitivity. Thus, it is fair to say anti-PF4 should be examined in post-COVID-19 vaccination thrombocytopenia to rule out VIT.
In the survey by Hippisley-Cox et al. [24], the association between COVID-19 vaccines and the risk of thrombocytopenia was examined. Accordingly, the risk ratio of thrombocytopenia after ChAdOx1 vaccination was 1.33 (95% CI, 1.19–1.47 at 8–14 days), and 5.27 (95% CI, 4.34–6.40 at 8–14 days) in SARS-CoV-2 infection. In the survey by Burn et al. [27], the increased risk of thrombocytopenia after ChAdOx1 vaccination was reported, while the other survey by the same first author did not show the increased risk [26]. As discussed in the post-vaccine thrombosis, the increased incidence of such rare complications can hardly be judged correctly.
Other vaccines
Thrombocytopenia after any vaccine can occur, however, a large-scale survey in Israel did not find the increased incidence of thrombocytopenia after BNT162b exposure compared to the matched control [28]. Whereas, Burn et al. [26] compared the rates of thrombocytopenia following vaccination with BNT162b2 and reported a ratio of 1.35 (95% CI, 1.30–1.41) after the first vaccination. After all, there are mixed results with regard to the increased prevalence of thrombocytopenia. Lee et al. [47] reported twenty cases of thrombocytopenia following mRNA vaccines. The authors concluded that even if these cases were causally related to prior vaccination, the estimated frequency was in the order of 1 in 1000,000 vaccinated subjects.
Beyond VITT/TTS, other causes of thrombocytopenia, including vaccine-induced ITP, TTP, and other autoimmune thrombocytopenia, have been discussed. The vaccine-thrombocytopenia reports to the US Vaccine Adverse Event Reporting System (VAERS) evaluated thrombocytopenia patterns from 1990 to 2008 for differences in single versus multiple immunization reports, presence of a live viral vaccine, severity, age, and interval to symptom onset [48]. They found 1510 reports of thrombocytopenia, and after exclusions, examined 1440 reports for possible causes. A total of 75% (1078 cases) met the regulatory definition of a serious adverse event, occurred after inactivated and live viral vaccines, and were defined as platelet counts < 10 × 109 /L [48]. Since the post-vaccination thrombocytopenia can accompany bleeding and/or thrombosis, specific management along with additional studies of thrombocytopenia after vaccinations should be considered.
Differential diagnosis
The diagnosis of VITT/TTS is supported when the patients fulfill the following criteria: (1) onset of symptoms 5–30 days (rarely, up to 45 days) after vaccination, (2) presence of thrombosis, (3) thrombocytopenia, (4) D-dimer levels of more than 4000 fibrinogen-equivalent units (FEU), (5) positive for anti-PF4 antibodies on ELISA [49]. However, the diagnosis is not always easy, and some cases show confusing symptoms and test results. The fundamental problem is the immune derangement caused by vaccination, and nonpathogenic anti-PF4 antibody is produced in such cases. Therefore, even if the ELISA for anti-PF4 antibody is positive, it may not necessarily ensure a diagnosis of VITT/TTS. If the case features are otherwise not indicative of VITT/TTS, testing for platelet-activating antibodies may be useful (in this situation, sample referral to a center specializing in HIT platelet activation assays is required). A diagnostic algorithm that does not utilize referral for platelet activation assays is shown in Fig. 2 . VITT/TTS-mimicking disorders that feature the duad of thrombosis and thrombocytopenia are discussed subsequently.
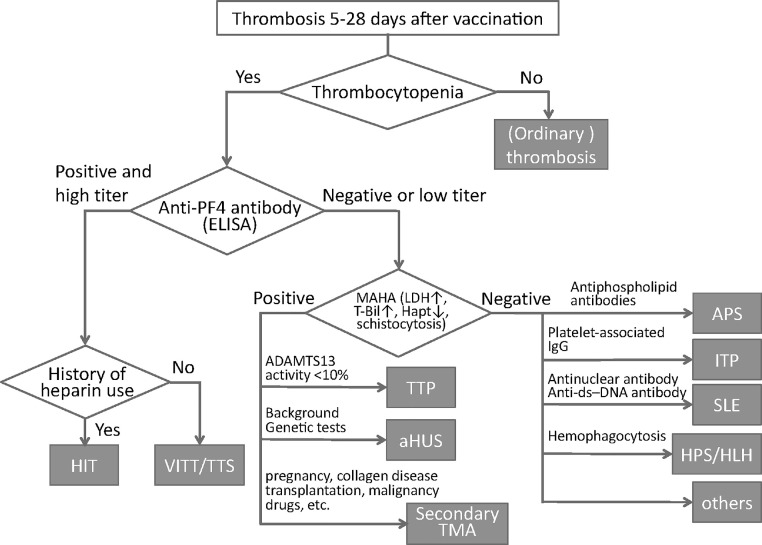
Fig. 2.
Algorithm to differentiate vaccine-induced thrombotic thrombocytopenia (VITT)/thrombosis with thrombocytopenia syndrome (TTS) from other thrombotic diseases. When the patients show thrombosis between 5 and 28 days after COVID-19 vaccination, the platelet count should be calculated. If patients show low platelet count, vaccine-induced thrombocytopenia (VITT)/thrombosis with thrombocytopenia syndrome (TTS) should be differentiated from other thrombotic diseases by measuring antiplatelet factor 4 (PF4) antibody. Heparin-induced thrombocytopenia (HIT) is suspected when the patients have a history of heparins. If anti-PF4 antibody is negative or low titer, the presence of microangiopathic hemolytic anemia (MAHA) should be examined by measuring lactate dehydrogenase (LDH), total-bilirubin (T-Bil), haptoglobin (Hapt). TTP: thrombotic thrombocytopenic purpura, aHUS: atypical hemolytic uremic syndrome, TMA: thrombotic microangiopathy, APS: antiphospholipid syndrome. ITP: immune thrombocytopenic purpura, ds-DNA: double strand-DNA, SLE: systemic lupus erythematosus, HPS: hemophagocytic syndrome, HLH: hemophagocytic lymphohistiocytosis.
Heparin-induced thrombocytopenia (HIT)
HIT is potentially a life and limb-threatening complication of heparin exposure that occurs in 0.1–5% of patients receiving therapeutic or prophylactic doses of heparin [50]. HIT is caused by platelet-activating antibodies of IgG class that recognize PF4/heparin complexes. The pathogenesis of VITT/TTS overlaps with a subtype of HIT known as autoimmune HIT, a particular type of HIT that features antibodies that can activate platelets even in the absence of heparin; indeed, some cases of autoimmune HIT occur in the absence of any preceding exposure to heparin (so-called “spontaneous” HIT syndrome). Whereas deep vein thrombosis/pulmonary embolism and arterial thrombus are common in HIT, CVST and splanchnic vein thrombosis are most often seen in VITT/TTS. The anti-PF4 ELISA tests are usually positive for both diseases but HIT rapid screening assays (e.g., latex immunoassay, chemiluminescence immunoassay, particle gel immunoassay) are generally positive only for HIT [51], indicating differences in target antigens on PF4 between HIT and VITT/TTS. Sangli et al. [52] reported a case with severe thrombocytopenia with massive pulmonary embolism 10 days after vaccination with mRNA-1273. Anti-PF4 ELISA was positive, raising the issue of whether this was a true case of VITT/TTS (which has otherwise not been reported following vaccination with an mRNA vaccine) or an exceptionally rare example of environmentally-triggered spontaneous HIT syndrome.
Similar to the VITT/TTS, the source of polyanion in autoimmune HIT is unclear. Recent studies have implicated anti-PF4 antibodies bridge two PF4 tetramers by non-heparin platelet-associated polyanions, i.e., chondroitin sulfate and polyphosphates in autoimmune HIT [53]. Since heparin may not worsen VITT/TTS, but it does on HIT, it will be helpful to differentiate them.
Antiphospholipid syndrome (APS)
APS is an autoimmune disorder characterized by arterial, venous, and/or small vessel thrombosis induced by antiphospholipid antibodies that include lupus anticoagulant, anticardiolipin, and anti-β2 Glycoprotein I antibodies. These antibodies are detectable in about half of critically ill COVID-19 patients and can cause secondary APS. However, in most cases, the presence of antiphospholipid antibodies is transient and disappears within a few weeks, and antibody titers are not high enough to develop thrombosis [54]. In addition, Sciascia et al. [55] reported that although antiphospholipid testing is positive in COVID-19, the antiphospholipid antibody profile differs from that seen in patients with symptomatic APS.
Regarding post-vaccine APS, a few cases of thrombocytopenia and thrombotic events resembling APS have been reported after vaccination with vector- and mRNA COVID-19 vaccines [56]. Both vectored vaccine and mRNA vaccine may confer a prothrombotic phenotype to platelets. Adenovirus can bind platelets and induce their destruction in the reticuloendothelial system, and mRNA vaccine can favor activation of coagulation and inflammation. In addition, both formulations may trigger a type I interferon response associated with the generation of antiphospholipid antibodies [56].
Misdiagnosis of APS as VITT or VITT as APS is also a potential concern after vaccination. For differentiating, although the antiphospholipid antigens are not highly specific, the diagnostic value of high levels of anti-PF4 antibodies for VITT may be helpful. However, clinicians should be cautious in interpreting serological testing for the diagnosis of VITT.
Thrombotic thrombocytopenic purpura (TTP)
TTP is a rare and life-threatening disease that features thrombocytopenia and microangiopathic hemolytic anemia together with deficiency of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) [57]. The clinical picture of TTP usually includes severe thrombocytopenia and organ ischemia/dysfunction and thus can clinically resemble VITT/TTS.
Multiple cases of COVID-19 complicated by TTP have been reported, but it may not always be easy to identify TTP in COVID-19 since such cases are quite rare and the ADAMTS-13 levels are often low in critical illness [58]. Thrombosis in TTP is typically formed in the arterioles due to platelet/von Willebrand factor microaggregates, and which lead to microangiopathic hemolytic anemia (MAHA). Thus, the key to diagnosis is the detection of MAHA represented by elevated lactate dehydrogenase (LD) and total bilirubin, decreased hemoglobin, decreased or absent haptoglobin, and peripheral blood presence of red blood cell fragments. In addition, since acquired TTP is caused by autoantibody-induced depletion or inhibition of ADAMTS13, use of a rapid ADAMTS13 activity assay is crucial [59], and the detection of ultra-large von Willebrand factor is also helpful for the diagnosis [60].
The immune derangement induced by either vectored or mRNA vaccination can cause newly developed or relapsed TTP [61,62]. Lately, a new pathologic insight of this unique hematologic disorder, TTP associated with COVID-19 mRNA vaccine, has been reported. Waqar et al. [63] suggested a possible presence of vaccinal antigens against ADAMTS13 specific gene responsible for creating a robust immune response.
Immune thrombocytopenic purpura (ITP)
Acquired ITP is a disorder that can lead to excessive bruising and bleeding but is rarely complicated by thrombosis. The pathogenesis is multifactorial, but one of the primary mechanisms is the specific response from CD4+ T cells activated against modified glycoprotein (GP)IIb-IIIa on activated platelets. Approximately 60% of the patients with ITP, GPIIb-IIIa, and/or GPIb-IX autoantigens were identified as ligands for antiplatelet autoantibodies (platelet-associated IgG) [64].
ITP has emerged as an important complication of COVID-19, and a series of cases were reported, and SARS-CoV-2-mediated immune dysregulation can be attributed to the underlying condition [65]. Regarding ITP after COVID-19 vaccination, VAERS reported that fifteen cases of thrombocytopenia, not all but includes ITP, were identified in over 18 million doses BNT162b2 vaccine and 13 cases in over 16 million doses of mRNA-1273 vaccine. The incidence was quite low, and 0.80 per million doses for both vaccines, which is not greater than the number of ITP cases expected [66]. The case number is even fewer, but cases of thrombocytopenia with negative anti-PF4 antibody after ChAdOx1 vaccination were reported [67]. Since the diagnostic accuracy of platelet-associated IgG is not high, those cases may be diagnosed as ITP without testing platelet-associated IgG. The differentiation of VIT from ITP is important, especially after the COVID-19 vaccination.
Systemic lupus erythematosus (SLE) and rheumatic diseases
Patients with SLE are at increased risk of thrombosis and/or thrombocytopenia, and the poor outcomes in COVID-19 patients with SLE are known [68]. There is a potential concern that COVID-19 vaccines may worsen SLE and induce coagulopathy, but so far, no significant safety concern for the use of COVID-19 vaccines in patients with SLE and other rheumatic diseases are reported [69]. Nevertheless, the differential diagnosis of SLE from VITT/TTS should be considered. Recently, a rare case of newly developed SLE with thrombosis and thrombocytopenia was reported [70]. Although the low sensitivity of the rapid test for anti-PF4 antibody in VITT/TTS has already been known, one should also take into account the potential risk of false-positive reactions of the rapid test and ELISA anti-PF4 antibody (e.g., cross-reacting antibodies and subjective interpretation of the test result). It is important to know that a significant number of SLE patients with or without APS yield false-positive anti-PF4 test results due to autoantibodies [71].
Hemophagocytic syndrome (HPS)/hemophagocytic lymphohistiocytosis (HLH)
HPS or HLH is a hyperinflammatory syndrome characterized by fever, splenomegaly, decreased counts in two cell lines, hypertriglyceridemia and/or hypofibrinogenemia, and hyper hemophagocytosis. Acquired HPS/HLH is due to large amounts of proinflammatory cytokines released from activated macrophages and lymphocytes secondary to various triggers, including viral infection [72]. The clinical symptoms and laboratory findings, such as increased IL-6 levels and hyperferritinemia, are common with COVID-19, and the partially overlapped pathophysiology is present [12]. Similarly, the association of VTTT/TTS and HPS/HLH is suggested, and Ricke [73] proposed the potential involvement of hemophagocytic histocytes targeting platelets bound by anti-PF4 autoantibodies.
Conclusions
Thrombosis and thrombocytopenia are often seen in severe COVID-19. Comparatively, VITT/TTS is a much rare event after vaccination with the vectored vaccine. Meanwhile, thrombosis and/or thrombocytopenia can be seen after vaccination with any COVID-19 vaccine since the spike protein activates macrophages and elicits inflammation, stimulates PF4 release from platelets, and downregulates ACE2 from the endothelial surface. This spike protein-induced inflammation is a possible explanation that causes COVID-19-associated coagulopathy, and thrombosis and/or thrombocytopenia after COVID-19 vaccination. However, it may not be sufficient to explain VITT/TTS because of its strong association with adenoviral vector vaccines.
For the management of VITT/TTS, early and accurate diagnosis is important, and the measurements of D-dimer and anti-PF4 antibody are useful for the initial evaluation. However, many diseases mimic VITT/TTS, such as HIT, APS, TTP, ITP, SLE, HPS/HLH, and some can show positive ELISA anti-PF4 antibodies. Therefore, the differential diagnosis is important if the symptoms or clinical courses are unusual.
We emphasize that although rare side effects can occur in COVID-19 vaccinations, the clear benefit of vaccines continues to be demonstrated compared to the risk of morbidity, mortality, and persistent debilitating effects of long COVID19 in unvaccinated patients.
Finally, this review discussed the association of thrombosis/thrombocytopenia and COVID-19, but there is little current information that examines the new variants of SARS-CoV-2, including omicron. Since the spike protein plays an essential role in platelet activation, the assessment for the new variants will be important for future studies.
Ethical statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that all authors are responsible for the content and have read and approved the manuscript; and that the manuscript conforms to the Uniform Requirements for Manuscripts Submitted to Biomedical Journals published in Annals in Internal Medicine.
We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Signed by all authors as follows:
Toshiaki Iba, MD, Jerrold H Levy.
PhD Jerrold Levy, MD, FCCM.
CRediT authorship contribution statement
Toshiaki Iba: Writing – original draft, Writing – review & editing. Jerrold H. Levy: Writing – review & editing.
Acknowledgments
We appreciate Professor Theodore E. Warkentin for his review and advice.
Footnotes
Funding: This work was supported in part by a Grant-in-Aid for Scientific Research C Grant Number JP19K09424.
Declaration of Competing Interest: TI has received a research grant from Japan Blood Products Organization and JIMRO. JHL serves on Steering Committees for Instrumentation Laboratories, Octapharma, and Merck.
References
- 1.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T., Levy J.H. The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune thrombotic thrombocytopenia. Trends Cardiovasc Med. 2021;S1050-1738(21):00096–00097. doi: 10.1016/j.tcm.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piazza G., Campia U., Hurwitz S., Snyder J.E., Rizzo S.M., Pfeferman M.B., et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T., Connors J.M., Levy J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69(12):1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta P.K., Liu F., Fischer T., Rappaport J., Qin X. SARS-CoV-2 pandemic and research gaps: understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448–7464. doi: 10.7150/thno.48076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y., Zhao J., Li J., Guo Z., Sheng J., Ye X., et al. SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate. Int J Biol Macromol. 2021;S0141-8130(21):02270–02274. doi: 10.1016/j.ijbiomac.2021.10.112. Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taquet M., Husain M., Geddes J.R., Luciano S., Harrison P.J. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. eClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iba T., Levy J.H., Connors J.M., Warkentin T.E., Thachil J., Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24(1):360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez van Kammen M., Heldner M.R., Brodard J., Scutelnic A., Silvis S., Schroeder V., et al. Frequency of thrombocytopenia and platelet factor 4/heparin antibodies in patients with cerebral venous sinus thrombosis prior to the COVID-19 pandemic. JAMA. 2021;326(4):332–338. doi: 10.1001/jama.2021.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krzywicka K., Heldner M.R., Sánchez van Kammen M., van Haaps T., Hiltunen S., Silvis S.M., et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European medicines agency. Eur J Neurol. 2021;28(11):3656–3662. doi: 10.1111/ene.15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharifian-Dorche M., Bahmanyar M., Sharifian-Dorche A., Mohammadi P., Nomovi M., Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 2021;428 doi: 10.1016/j.jns.2021.117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Althaus K., Möller P., Uzun G., Singh A., Beck A., Bettag M., et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106(8):2170–2179. doi: 10.3324/haematol.2021.279000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh A., Kelton J.G., Arnold D.M., Daka M., Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596(7873):565–569. doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 19.McGonagle D., De Marco G., Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun. 2021;121 doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greinacher A., Selleng K., Palankar R., Wesche J., Handtke S., Wolff M., et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(22):2256–2268. doi: 10.1182/blood.2021013231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greinacher A., Selleng K., Mayerle J., Palankar R., Wesche J., Reiche S., et al. Antiplatelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood. 2021;138(14):1269–1277. doi: 10.1182/blood.2021012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hippisley-Cox J., Patone M., Mei X.W., Saatci D., Dixon S., Khunti K., et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers E.R., McClure D.L., Naleway A.L., Jacobsen S.J., Klein N.P., Glanz J.M., et al. Risk of venous thromboembolism following influenza vaccination in adults aged 50 years and older in the vaccine safety datalink. Vaccine. 2017;35(43):5872–5877. doi: 10.1016/j.vaccine.2017.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burn E, Roel E, Pistillo A, Fernandez-Bertolí S, Aragón M, Carlen Reyes C, et al. Thromboembolic events and thrombosis with thrombocytopenia after COVID-19 infection and vaccination in Catalonia, Spain. https://www.medrxiv.org/content/10.1101/2021.07.29.21261348v1.
- 27.Burn E, Li X, Delmestri A, Jones N, Duarte-Salles T, Reyes C, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2: a population-based cohort analysis. doi:10.1101/2021.07.29.21261348v1 [DOI] [PMC free article] [PubMed]
- 28.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiele T., Ulm L., Holtfreter S., Schönborn L., Kuhn S.O., Scheer C., et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138(4):299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiore M.M., Kakkar V.V. Platelet factor 4 neutralizes heparan sulfate-enhanced antithrombin inactivation of factor Xa by preventing interaction(s) of enzyme with polysaccharide. Biochem Biophys Res Commun. 2003;311(1):71–76. doi: 10.1016/j.bbrc.2003.09.171. [DOI] [PubMed] [Google Scholar]
- 31.Comer S.P., Cullivan S., Szklanna P.B., Weiss L., Cullen S., Kelliher S., et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19(2) doi: 10.1371/journal.pbio.3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demma L., Levy J.H. A case series of recombinant platelet factor 4 for heparin reversal after cardiopulmonary bypass. Anesth Analg. 2012;115(6):1273–1278. doi: 10.1213/ANE.0b013e3182662e1a. [DOI] [PubMed] [Google Scholar]
- 33.Palankar R., Kohler T.P., Krauel K., Wesche J., Hammerschmidt S., Greinacher A. Platelets kill bacteria by bridging innate and adaptive immunity via platelet factor 4 and FcγRIIA. J Thromb Haemost. 2018;16(6):1187–1197. doi: 10.1111/jth.13955. [DOI] [PubMed] [Google Scholar]
- 34.Stuart L.M. In gratitude for mRNA vaccines. N Engl J Med. 2021;385(15):1436–1438. doi: 10.1056/NEJMcibr2111445. [DOI] [PubMed] [Google Scholar]
- 35.Azzarone B., Veneziani I., Moretta L., Maggi E. Pathogenic mechanisms of vaccine-induced immune thrombotic thrombocytopenia in people receiving anti-COVID-19 adenoviral-based vaccines: a proposal. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.728513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maquet J., Lafaurie M., Sommet A., Moulis G. Thrombocytopenia is independently associated with poor outcome in patients hospitalized for COVID-19. Br J Haematol. 2020;190(5):e276–e279. doi: 10.1111/bjh.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soy M., Atagündüz P., Atagündüz I., Sucak G.T. Hemophagocytic lymphohistiocytosis: a review inspired by the COVID-19 pandemic. Rheumatol Int. 2021;41(1):7–18. doi: 10.1007/s00296-020-04636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazy I., Jevtic S.D., Moore J.C., Huynh A., Smith J.W., Kelton J.G., Arnold D.M. Platelet-activating immune complexes identified in critically ill COVID-19 patients suspected of heparin-induced thrombocytopenia. J Thromb Haemost. 2021;19(5):1342–1347. doi: 10.1111/jth.15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maharaj S., Chang S. Anti-PF4/heparin antibodies are increased in hospitalized patients with bacterial sepsis. Thromb Res. 2018;171:111–113. doi: 10.1016/j.thromres.2018.09.060. [DOI] [PubMed] [Google Scholar]
- 40.Cacciola R., Gentilini Cacciola E., Vecchio V., Cacciola E. Cellular and molecular mechanisms in COVID-19 coagulopathy: role of inflammation and endotheliopathy. J Thromb Thrombolysis. 2021:1–9. doi: 10.1007/s11239-021-02583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delrue M., Siguret V., Neuwirth M., Brumpt C., Voicu S., Burlacu R., et al. Contrast between prevalence of HIT antibodies and confirmed HIT in hospitalized COVID-19 patients: a prospective study with clinical implications. Thromb Haemost. 2021;121(7):971–975. doi: 10.1055/a-1333-4688. [DOI] [PubMed] [Google Scholar]
- 42.Riker R.R., May T.L., Fraser G.L., Gagnon D.J., Bandara M., Zemrak W.R., Seder D.B. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res Pract Thromb Haemost. 2020;4(05):936–941. doi: 10.1002/rth2.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patell R., Khan A.M., Bogue T., Merrill M., Koshy A., Bindal P., et al. Heparin induced thrombocytopenia antibodies in COVID-19. Am J Hematol. 2020 doi: 10.1002/ajh.25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wester J.P., Haas F.J., Biesma D.H., Leusink J.A., Veth G. Thrombosis and hemorrhage in heparin-induced thrombocytopenia in seriously ill patients. Intensive Care Med. 2004;30(10):1927–1934. doi: 10.1007/s00134-004-2334-1. [DOI] [PubMed] [Google Scholar]
- 45.Salih F., Schönborn L., Kohler S., Franke C., Möckel M., Dörner T., et al. Vaccine-induced thrombocytopenia with severe headache. N Engl J Med. 2021 doi: 10.1056/NEJMc2112974. doi: NEJMc2112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuter D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021;195(3):365–370. doi: 10.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee E.J., Cines D.B., Gernsheimer T., et al. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo E.J., Wise R.P., Menschik D., Shadomy S.V., Iskander J., Beeler J., Varricchio F., Ball R. Thrombocytopenia after vaccination: case reports to the US vaccine adverse event reporting system, 1990-2008. Vaccine. 2011;29(6):1319–1323. doi: 10.1016/j.vaccine.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 49.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogan M., Berger J.S. Heparin-induced thrombocytopenia (HIT): review of incidence, diagnosis, and management. Vasc Med. 2020;25(2):160–173. doi: 10.1177/1358863X19898253. [DOI] [PubMed] [Google Scholar]
- 51.Iba T., Levy J.H., Warkentin T.E. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sangli S., Virani A., Cheronis N., Vannatter B., Minich C., Noronha S., et al. Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine. Ann Intern Med. 2021;174(10):1480–1482. doi: 10.7326/L21-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greinacher A., Selleng K., Warkentin T.E. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 54.Xiao M., Zhang Y., Zhang S., Qin X., Xia P., Cao W., et al. Antiphospholipid antibodies in critically Ill patients with COVID-19. Arthritis Rheumatol. 2020;72(12):1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sciascia S., Radin M., Bazzan M., Montaruli B., Cosseddu D., Norbiato C., et al. Antiphospholipid antibodies and infection: non nova sed nove. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.687534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talotta R., Robertson E.S. Antiphospholipid antibodies and risk of post-COVID-19 vaccination thrombophilia: the straw that breaks the camel's back? Cytokine Growth Factor Rev. 2021;60:52–60. doi: 10.1016/j.cytogfr.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joly B.S., Coppo P., Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–2846. doi: 10.1182/blood-2016-10-709857. [DOI] [PubMed] [Google Scholar]
- 58.Altowyan E., Alnujeidi O., Alhujilan A., Alkathlan M. COVID-19 presenting as thrombotic thrombocytopenic purpura (TTP) BMJ Case Rep. 2020;13(12) doi: 10.1136/bcr-2020-238026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas W., Cutler J.A., Moore G.W., McDonald V., Hunt B.J. The utility of a fast turnaround ADAMTS13 activity in the diagnosis and exclusion of thrombotic thrombocytopenic purpura. Br J Haematol. 2018 doi: 10.1111/bjh.15219. [DOI] [PubMed] [Google Scholar]
- 60.Favaloro E.J., Pasalic L., Henry B., Lippi G. Laboratory testing for ADAMTS13: utility for TTP diagnosis/exclusion and beyond. Am J Hematol. 2021;96(8):1049–1055. doi: 10.1002/ajh.26241. [DOI] [PubMed] [Google Scholar]
- 61.Lee H.P., Selvaratnam V., Rajasuriar J.S. Thrombotic thrombocytopenic purpura after ChAdOx1 nCoV-19 vaccine. BMJ Case Rep. 2021;14(10) doi: 10.1136/bcr-2021-246049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maayan H., Kirgner I., Gutwein O., Herzog-Tzarfati K., Rahimi-Levene N., Michowitz M., et al. Acquired thrombotic thrombocytopenic purpura: a rare disease associated with BNT162b2 vaccine. J Thromb Haemost. 2021;19(9):2314–2317. doi: 10.1111/jth.15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waqar S.H.B., Khan A.A., Memon S. Thrombotic thrombocytopenic purpura: a new menace after COVID bnt162b2 vaccine. Int J Hematol. 2021;114(5):626–629. doi: 10.1007/s12185-021-03190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coopamah M.D., Garvey M.B., Freedman J., Semple J.W. Cellular immune mechanisms in autoimmune thrombocytopenic purpura: an update. Transfus Med Rev. 2003;17(1):69–80. doi: 10.1053/tmrv.2003.50004. [DOI] [PubMed] [Google Scholar]
- 65.Bhattacharjee S., Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Compr Clin Med. 2020;2(11):2048–2058. doi: 10.1007/s42399-020-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welsh K.J., Baumblatt J., Chege W., Goud R., Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the vaccine adverse event reporting system (VAERS) Vaccine. 2021;39(25):3329–3332. doi: 10.1016/j.vaccine.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uaprasert N., Panrong K., Tungjitviboonkun S., Dussadee K., Decharatanachart P., Kaveevorayan P., et al. ChAdOx1 nCoV-19 vaccine-associated thrombocytopenia: three cases of immune thrombocytopenia after 107 720 doses of ChAdOx1 vaccination in Thailand. Blood Coagul Fibrinolysis. 2021 doi: 10.1097/MBC.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 68.Mason A., Anver H., Lwin M., Holroyd C., Faust S.N., Edwards C.J. Lupus, vaccinations and COVID-19: what we know now. Lupus. 2021;30(10):1541–1552. doi: 10.1177/09612033211024355. [DOI] [PubMed] [Google Scholar]
- 69.Tang W., Gartshteyn Y., Ricker E., Inzerillo S., Murray S., Khalili L., et al. The use of COVID-19 vaccines in patients with SLE. Curr Rheumatol Rep. 2021;23(11):79. doi: 10.1007/s11926-021-01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Bruyne S., Degandt S., Ghys T., Louagie H. Thrombocytopenia after coronavirus disease 2019 vaccination: remember to put the blame on others too. Crit Care Med. 2021 doi: 10.1097/CCM.0000000000005327. [DOI] [PubMed] [Google Scholar]
- 71.Pauzner R., Greinacher A., Selleng K., Althaus K., Shenkman B., Seligsohn U. False-positive tests for heparin-induced thrombocytopenia in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Thromb Haemost. 2009;7:1070–1074. doi: 10.1111/j.1538-7836.2009.03335.x. [DOI] [PubMed] [Google Scholar]
- 72.Ramachandra S., Zaidi F., Aggarwal A., Gera R. Recent advances in diagnostic and therapeutic guidelines for primary and secondary hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2017;64:53–57. doi: 10.1016/j.bcmd.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 73.Ricke D.O. Models for SARS-CoV-2 associated thrombocytopenia associated with hemophagocytic histiocytes. Med Hypotheses. 2021;157 doi: 10.1016/j.mehy.2021.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]