Abstract
The concept of a direct association between coronary graft patency and clinical status is generally accepted. However, the relationship is more complex and variable than usually thought. Key issues are the lack of a common definition of graft occlusion and of a standardized imaging protocol for patients undergoing coronary bypass surgery. Factors like the type of graft, the timing of the occlusion, and the amount of myocardium at risk, as well as baseline patients’ characteristics, modulate the patency-to-clinical status association. Available evidence suggests that graft occlusion is more often associated with non-fatal events rather than death. Also, graft failure due to competitive flow is generally a benign event, while graft occlusion in a graft-dependent circulation is associated with clinical symptoms. In this systematic review, we summarize the evidence on the association between graft status and clinical outcomes.
Keywords: Patency, CABG, Graft failure
Graphical Abstract
Introduction
The classic concept of a direct and close association between coronary artery graft patency and clinical status is intuitive and biologically plausible. However, the relationship is more complex and variable than generally thought. In this review, we summarize the evidence of the association between coronary graft patency and clinical status in patients with coronary artery disease. A systematic review of the literature was performed by a medical librarian; the search strategy, study selection methods, and the PRISMA flowchart are provided in the Supplementary material online (Supplementary material online, Table S1 and Figure S1).
Incidence, mechanisms, and timing of graft failure
The incidence of graft failure varies for the different types of conduits. At 5 years, failure rates of 17.5% for the saphenous vein graft (SVG), 2.3% and 13.5% for the left and right internal thoracic artery (LITA and RITA), respectively, and 9.4% for the radial artery (RA) have been reported.1 , 2 Ten-year failure rates are 39% for SVG, 15% for LITA, 20–25% for RITA, and 11–15% for the RA.3 , 4
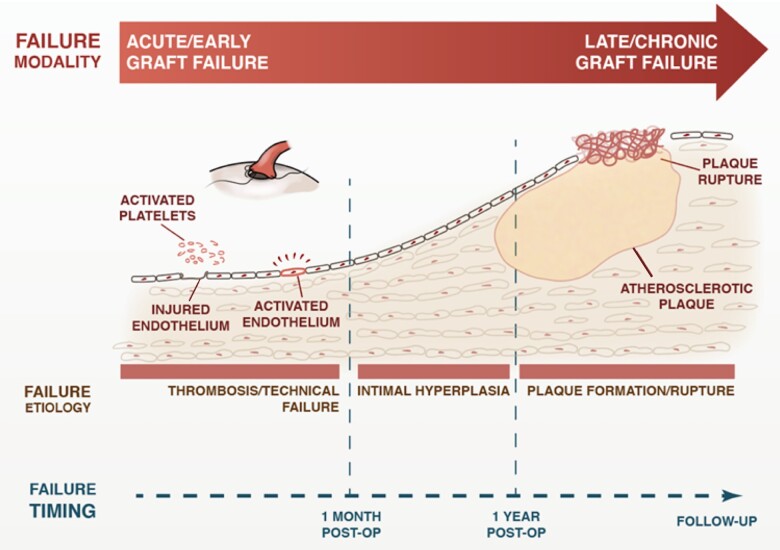
The pathophysiology of graft failure varies in different postoperative periods, being mainly due to acute thrombosis within the first postoperative month, intimal hyperplasia in the 1st year, and atherosclerosis thereafter (Figure 1).1
Figure 1.
Pathophysiology of graft failure in different postoperative periods.
Early failure is predominantly due to technical factors (anastomotic defect, kinking), competitive flow, haemodynamic factors,5 or hypercoagulability.6 Intimal hyperplasia is an adaptive process that starts at the anastomotic site and is generally self-limited, but in some cases, can become generalized and lead to graft occlusion.7 Atherosclerosis in bypass grafts is accelerated compared with native coronary arteries and has distinctive morphologic features such as a concentric and diffuse pattern and a poorly defined fibrous cap at high risk for rupture.8
The different mechanisms lead to failure with different time courses: technical failure and thrombosis usually cause acute occlusion, while failure due to intimal hyperplasia and atherosclerosis occurs over a longer period of time.
Different types of bypass conduits have different histologic characteristics of failure, with fibrous hyperplasia being more common in arterial grafts and atherosclerosis in SVGs. Sex and other baseline clinical characteristics,9 pharmacological interventions, such as the use of anti-platelets and lipid-lowering therapy,10 , 11 and even genetic variants12 may affect the risk of graft failure.
Current guidelines recommend that early graft failure is treated by either percutaneous interventions or reoperation, based on the Heart Team decision, while percutaneous interventions are preferred to treat late graft failure.13
The issues with graft patency studies
Invasive angiography has traditionally been used for patency studies, but we now have evidence that computed tomography angiography has very high sensitivity and specificity in assessing coronary grafts14 and will likely become the new standard. The use of intravascular imaging techniques adds important anatomic information and may help in identifying the mechanism responsible for graft failure.15
A key factor complicating the analysis of graft patency studies is the lack of a universal definition of graft failure and of graft patency. Although in recent years, the three-level Fitzgibbon classification (grade A: excellent graft with unimpaired runoff; grade B: stenosis reducing calibre of proximal or distal anastomoses or trunk to <50% of the grafted coronary artery; grade O: occlusion)16 has been adopted by several groups, many investigators have used different scales with heterogeneous definitions.17
Another key issue is that from graft patency studies one can determine only when graft occlusion was visualized, but not when occlusion occurred. In trying to establish a causal relationship between graft failure and clinical events, it is key to know that graft failure came first and the event thereafter.
Other important complicating factors are differences in the completeness and timing of follow-up in the published series.17 Graft patency studies inherently suffer from survivor bias. In most observational series, imaging of grafts is reserved for symptomatic patients introducing a major selection bias. It has been shown that reported graft failure rates are higher in studies including only symptomatic patients.18 Even in randomized controlled trials, incomplete follow-up is common because of new medical complications or consent withdrawal. It has been shown that patients who decline late angiography have different baseline risk profiles than patients with imaging follow-up.19 While analytic options to address incomplete follow-up exist,20 they have not been consistently applied.
When femoral access and use of angiography were the norm, the selection criteria for graft patency studies excluded patients with vascular and renal disease. This limitation is less relevant today, but it applies to most of the published evidence. An important consideration is patient age—older patients represent a large proportion of patients undergoing surgical revascularization but because of their shorter survival and higher comorbidities are under-represented in patency studies.
Another important confounding factor is related to the functional severity of the grafted coronary vessel.21 Functional data have shown that when the indication to revascularization is based on angiography alone, up to 20% of the grafted target vessels do not have flow-limiting stenosis.22 , 23 The consequent chronic native competitive flow is a strong risk factor for occlusion for arterial grafts, and among them the RA is more affected than the internal thoracic artery24 , 25 (Figure 2). However, graft occlusion due to competitive flow is a different event than graft occlusion from other causes. In the IMPAG trial, arterial graft failure was mainly due to competitive flow and was not associated with clinical events.24 SYNTAX-LE MANS was an angiographic sub-study of the SYNTAX trial evaluating the patency of bypass grafts in patients with left main disease, a situation at high risk of competitive flow: no correlation between adverse events at 15-month follow-up and graft failure was found.26
Figure 2.
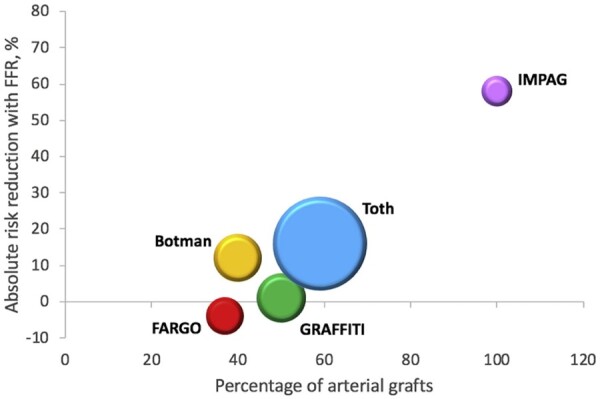
Reduction in graft failure using fractional flow reserve for coronary artery bypass grafting. Reproduced with permission from Spadaccio et al. 25
The likely reason behind this finding is that the implantation of a bypass graft creates a separate coronary inflow, parallel to the native circulation. If the latter is dominant and the graft fails from competitive flow, there is no reduction in distal perfusion and, thus, no clinical events associated with graft failure. In contrast, with percutaneous interventions and stent thrombosis, there is a sudden reduction of the only flow source that generally results in clinical events.
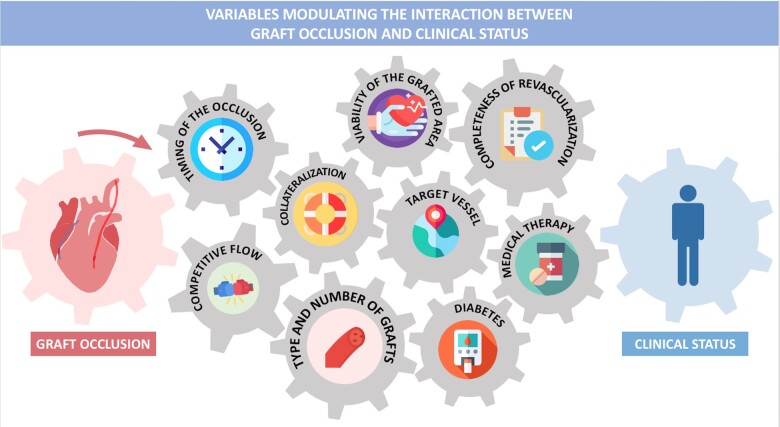
The completeness of revascularization and the number of grafts implanted, as well as the viability of the myocardial area served by the graft are other important modulators of the association of graft occlusion with clinical events (Graphical abstract).
Variables modulating the interaction between graft occlusion and clinical status.
In addition, some of the factors that affect graft patency, such as renal failure and diabetes, also affect progression of native coronary disease, and clinical outcomes may be driven by the latter rather than the former.
Finally, it must be noted that the majority of patency studies date back to the era of the introduction of coronary surgery and may not represent current surgical practice (Table 1).16 , 26–38
Table 1.
Overview of selected studies examining the relationship between graft patency and clinical outcomes after coronary artery bypass grafting
| Study (first author, year) | Type of study | Total no. of CABG patients | No. of CABG patients who underwent imaging follow-up | Indication for imaging follow-up | Completeness of imaging follow-up | Follow-up time (mean/ median, years) | Type of graft studied | Key findings |
|---|---|---|---|---|---|---|---|---|
| Studies showing an association between graft patency and clinical outcomes | ||||||||
| Bourassa, 198228 | Prospective | 600 | 108 | Per-protocol angiography | 18.0% | 6 | SVG | Graft occlusion was significantly associated with recurrence of angina. Graft patency was significantly associated with survival. |
| Laird-Meeter, 198329 | Prospective | 1041 | 169 | Per-protocol angiography | 16.2% | 3.5 | SVG | Graft occlusion was significantly associated with recurrence of angina. |
| Lytle, 199231 | Retrospective | NR | 1296 | Clinically driven angiography | NR | 6.9 | SVG | Late (≥5 years after CABG) stenosis in grafts to the LAD was associated with decreased survival, decreased reoperation-free survival, and decreased event-free survival (P < 0.001 for all), whereas early (<5 years after CABG) graft stenosis did not influence outcome. |
| Fitzgibbon, 199616 | Prospective | 1388 | NR | Per-protocol angiography | NR (of the total 5284 original grafts, 1987 were studied at 5 years) | Up to 22.5 | SVG | Vein graft failure was associated with increased reoperation rate and decreased survival. |
| Knatterud, 200333 | Substudy—the POST CABG trial | 1351 | 961 | Per-protocol angiography | 71.1% | 3.4 | SVG | Progression of atherosclerosis in ≥1 graft was associated with an increased risk of adverse cardiac outcomes (RR 2.4, 95% CI 1.7–3.5), cardiovascular death or MI (RR 2.2, 95% CI 1.3–3.8), and revascularization (RR 3.3, 95% CI 2.0–5.3) (P < 0.001 for all). |
| Halabi, 200534 | Retrospective | 6745 | 1243 | Clinically driven angiography | 18.4% | 6.7 (IQR 3.0–11.5) | SVG | Critical, non-occlusive (75–99%) graft stenosis was the strongest predictor of the composite of death/MI/revascularization (HR 2.36, 95% CI 2.00–2.79, P < 0.0001). |
| Lopes, 201235 | Post hoc analysis— PREVENT IV trial | 2400 | 1829 | Per-protocol angiography | 76.2% | 4 | SVG | Vein graft failure was associated with the composite of death/MI/revascularization (HR 1.58, 95% CI 1.21–2.06, P = 0.008), but not with death (HR 1.04, 95% CI 0.71–1.52, P = 0.85), or death/MI (HR 1.08, 95% CI 0.77–1.53, P = 0.65). |
| Shavadia, 201536 | Retrospective | 5276 | 281 | Clinically driven angiography | 5.3% | 5.4 | SVG and arterial grafts | Patients with ≥70% ITA stenosis had a strong trend towards worse long-term survival compared with patients with vein graft failure (adjusted HR 2.2, 95% CI 0.98–5.0, P = 0.056). |
| Harskamp, 201637 | Post hoc analysis— PREVENT IV trial | 2400 | 1539 | Per-protocol angiography | 64.1% | 5.0 ± 0.7 | ITA | ITA failure was associated with the composite of death/MI/revascularization (HR 3.92, 95% CI 2.30–6.68, P < 0.0001), but not with death (HR 1.10, 95% CI 0.50–2.39, P = 0.82), or death/MI (HR 1.29, 95% CI 0.66–2.50, P = 0.46). |
| Yamasaki, 201638 | Post hoc analysis— RAPS trial | 529 | 234 | Both per-protocol and clinically driven angiography | 44.2% | 7.5 ± 1.3 | SVG and RA | Patients with significant (≥50%) graft stenosis had higher rates of the composite of death/MI/revascularization (P < 0.0001), and revascularization (P < 0.0009). |
| Studies showing no association between graft patency and clinical outcomes | ||||||||
| Robert, 197827 | Prospective | 117 | 19 | Per-protocol angiography | 16.2% | 6 | SVG | Progression of native coronary artery disease, but not graft occlusion, was significantly associated with recurrence of angina. |
| Brindis, 198430 | Prospective | NR | 18 | Per-protocol CT scan | NR | 4 to 9 days post-operatively | SVG | Perioperative MI was not associated with graft occlusion. |
| Huikuri, 199232 | Prospective | NR | 339 | Per-protocol angiography | NR | 5 | SVG | The presence of ≥1 occluded graft at 3 months after CABG was not associated with 5-year mortality. |
| Morice, 201126 | Substudy— SYNTAX- LE MANS | 348 | 115 | Per-protocol angiography | 33.0% | 1.3 | SVG and arterial grafts | In patients with left main disease the composite of death/MI/stroke/revascularization was not significantly associated with graft occlusion (P = 0.85). |
CABG, coronary artery bypass grafting; CI, confidence interval; CT, computed tomography; HR, hazard ratio; ITA, internal thoracic artery; LAD, left anterior descending artery; MI, myocardial infarction; NR, not reported; POST CABG, Post Coronary Artery Bypass Graft trial; PREVENT IV, Project of Ex-vivo Vein Graft Engineering via Transfection trial; RA, radial artery; RAPS, Radial Artery Patency Study; RR, relative risk; SVG, saphenous vein graft; SYNTAX-LE MANS, Synergy between PCI with TAXUS express and cardiac surgery left main angiographic sub-study.
Studies supporting an association between graft patency and clinical outcomes
In one of the first angiographic series of coronary artery bypass graft surgery (CABG), Winer et al. 39 performed postoperative angiography in 67 patients and found that angina improvement and graft patency were highly correlated. Bourassa et al. 28 described a close association between graft patency and survival in 600 CABG cases and Knatterud et al.33 in a sub-analysis of the POST CABG study showed that SVG failure was associated with adverse clinical events, including death.
Fitzgibbon et al.16 in a study of more than 5000 grafts found that graft patency was inversely associated with the need for reoperation and directly associated with survival. In an analysis from the Duke Cardiovascular Databank on 1243 patients, graft failure was associated with death, myocardial infarction (MI), or repeat revascularization.34
In a study of 1296 CABG patients, Lytle et al. 31 found that at 7-year follow-up, patients with SVG stenosis occurring within 5 years of surgery and patients with no SVG stenosis had similar outcomes, but when the stenosis occurred in the SVG to the left anterior descending artery (LAD), survival was significantly reduced and the adverse event rate increased.
Shavadia et al. 36 in a provincial angiographic database from Alberta found that LITA-to-LAD failure, but not SVG failure to non-LAD targets was associated with reduced long-term survival.
Loop et al. 40 found a significant association of decreased mortality and all cardiac endpoints with the use of the LITA compared with the SVG to graft the LAD, and postoperative angiography revealed significantly higher patency of the arterial conduit. A small randomized trial found similar results: improved cardiac event-free survival at 10 years in patients who received LITA vs. the SVG to graft the LAD, and improved patency of the arterial graft.41
In the PREVENT IV trial, graft failure was associated with an increased risk of revascularization, but not of death or MI and the association was consistent for venous and arterial grafts.35 Of note, most of the revascularizations were performed within 2 weeks of the protocol-mandated angiography, suggesting that the event rate may have been inflated due to the imaging protocol.
In the RAPS trial, the risk of adverse events was significantly higher in patients with graft stenosis.38 An individual patient-level meta-analysis of six angiographic trials comparing RA with SVG found a reduction in graft occlusion and in the composite of death, MI, or repeat revascularization at 5 years in the RA arm.42 An association between graft patency and stress test performance was reported by Korpilahti et al.43 in 1999.
The use of cholesterol-lowering medications and of anti-thrombotic or anti-platelet therapy reduced SVG occlusion and improved outcomes in several trials, but the causal relation between patency and outcomes is unclear due to the effect of the medications on the native coronary circulation.11 , 44 , 45
Studies showing no association between graft patency and clinical outcomes
Robert et al. 27 in a study of 72 CABG patients found that at long-term follow-up, angina recurrence was mainly due to progression of native disease rather than graft occlusion. Achuff et al.46 described symptomatic and functional improvement in 7 out of 12 patients with all grafts occluded and Benchimol et al. 47 did not find any association between clinical status and graft patency in a small series of 32 patients.
Hoel et al. 48 in a study of 90 patients found no association between graft patency and MI and Huikuri et al. 32 in a study of 339 patients showed that graft patency was not associated with survival at 5-year follow-up.
In the ROOBY trial, LAD status, but not graft patency, was associated with patient-reported angina 1 year after CABG.49
In the acute postoperative setting, Brindis et al. 30 showed that the majority of the cases of perioperative MI after CABG were not associated with graft occlusion and Aintablian et al.50 did not find an association between graft status after surgery and postoperative appearance of new Q waves. A systematic review of the results of emergency angiography in patients with perioperative MI following CABG found that graft failure was not the cause of the acute event in 37.9% of the cases.51
Possible reasons for the described discrepancy among studies
While there is no clear-cut explanation for the reported discrepancy among studies, several factors need to be taken into account. Many of the series that show no association between graft failure and symptoms are from an era when it was common to graft any stenosis >50% in any target vessel; graft failure due to flow competition or limited run-off in small target vessels (both situations at low risk of clinical events) were likely more common in these series. In addition, routine postoperative imaging was used more commonly in the older series, while the more contemporary studies may suffer from a higher selection bias, with only symptomatic patients referred for imaging.
Effect of surgical grafting on native coronary circulation
Saphenous vein graft grafting has been shown to accelerate progression of the proximal native stenosis to occlusion in many studies.52–54 However, the use of arterial grafts is not associated with native stenosis progression and seems to have a protective effect on the distal coronary circulation (Table 2).52 , 55–58 While the mechanisms of this effect are speculative, it is possible that the local anti-inflammatory and antithrombotic molecules that prevent atherosclerosis in arterial grafts may exert a protective effect on the native coronary bed.
Table 2.
Overview of selected studies examining the effect of CABG graft type on disease progression in the native coronary circulation
| Study (first author, year) | Type of study | No. of patients included | Follow-up (mean/median, years) | Comparators | Key finding |
|---|---|---|---|---|---|
| Alderman, 199357 | Post hoc analysis— CASS study | 314 | 5 | LITA vs. SVG | A significant increase in native coronary artery disease progression in the LAD territory was observed in patients who received an SVG instead of an LITA graft. |
| Dimitrova, 201255 | Observational | 772 | 5.5 ± 3.5 | LITA vs. RA vs. SVG | RA and LITA grafting had a strong protective effect against progression of native coronary artery disease. Native vessel disease progression at 1, 5, and 10 years after CABG was 0.01%, 4%, and 8% in territories with patent LITA grafts; 0.01%, 6%, and 11% with patent RA grafts (P = 0.157); and 3%, 19%, and 43% with patent SVG (P < 0.0001). |
| Zhu, 201458 | Post hoc analysis— RAPCO trial | 405 | 6.2 ± 3.1 | Arterial graft vs. SVG | The use of arterial grafts was an independent predictor of disease regression in the native vessel. |
| Zhang, 201656 | Observational | 468 |
5.4 ± 3.4 (CABG) 5.3 ± 3.4 (PCI) |
CABG vs. PCI | Patients receiving LITA-to-LAD CABG had a significantly lower incidence of downstream disease progression compared with those receiving LAD PCI with BMS or DES (LITA 12.4% vs. BMS 85.9%, HR 0.34, 95% CI 0.20–0.59; vs. DES 24.1%, HR 0.39, 95% CI 0.20–0.79). |
| Yoon, 201752 | Observational | 911 | 4.7 | Arterial graft vs. SVG vs. No graft | The new occlusion rate of vessels after CABG was highest with SVG, followed by arterial grafts, and lowest in non-bypassed vessels, irrespective of baseline vessel stenosis degree (intermediate stenosis, 11.1% vs. 5.2% vs. 1.7%, P < 0.001; severe stenosis, 23.7% vs. 15.9% vs. 9.9%, P < 0.001). |
BMS, bare metal stent; CABG, coronary artery bypass grafting; DES, drug-eluting stent; HR, hazard ratio; LAD, left anterior descending artery; LITA, left internal thoracic artery; PCI, percutaneous coronary intervention; RA, radial artery; SVG, saphenous vein graft.
Conclusions and practical implications
The association between graft patency and clinical status is complex and highly variable (Graphical abstract).
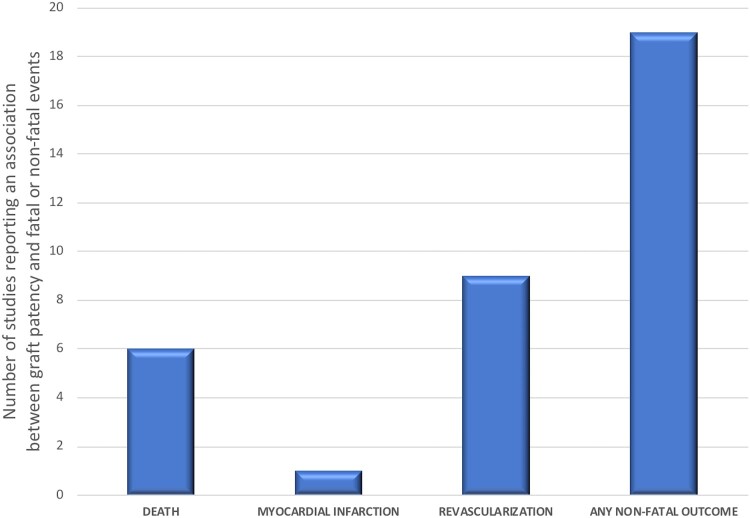
Overall, the number of studies that support an association between graft occlusion and clinical events (mostly non-fatal) is higher than the number of studies that refute it (Figure 3). An important exception is graft failure from competitive flow that is usually asymptomatic.
Figure 3.
Number of published studies reporting an association between graft patency and fatal or non-fatal events. As shown, graft occlusion is generally associated with non-fatal events rather than death.
Based on our data, one should conclude that the role of routine imaging follow-up after CABG appears limited. In addition, the incidental finding of asymptomatic graft failure in a CABG patient should not prompt re-intervention. Our finding of a variable association between graft status and clinical events has also implications for the design of future CABG trials: the assessment of clinical outcomes alone may underestimate the effect of interventions aimed at modifying graft patency and, for this reason, the use of patency as a surrogate or secondary outcome should be considered.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgements
The authors gratefully acknowledge Dr Malak Elbatarny’s assistance with figure editing.
Supplementary Material
Contributor Information
Mario Gaudino, Department of Cardiothoracic Surgery, Weill Cornell Medicine, 525 E 68th St, New York, NY 10065, USA.
Antonino Di Franco, Department of Cardiothoracic Surgery, Weill Cornell Medicine, 525 E 68th St, New York, NY 10065, USA.
Deepak L Bhatt, Heart and Vascular Center, Brigham and Women's Hospital, Harvard Medical School, 70 Francis St, Boston, MA 02115, USA.
John H Alexander, Duke Clinical Research Institute, Duke University Medical Center, 2400 Pratt St, Durham, NC 27705, USA.
Antonio Abbate, Division of Cardiology, VCU Pauley Heart Center and Wright Center for Clinical and Translational Research, Virginia Commonwealth University, 1200 E Marshall St, Richmond, VA 23219, USA.
Lorenzo Azzalini, Division of Cardiology, VCU Health Pauley Heart Center, Virginia Commonwealth University, 1200 E Marshall St, Richmond, VA 23219, USA.
Sigrid Sandner, Department of Cardiac Surgery, Medical University of Vienna, Währinger Gürtel 18-20, Vienna 1090, Austria.
Garima Sharma, Division of Cardiology, Johns Hopkins Ciccarone Center for Prevention of Cardiovascular Diseases, Johns Hopkins University School of Medicine, 1800 Orleans St, Baltimore, MD 21287, USA.
Sunil V Rao, Duke Clinical Research Institute, Duke University Medical Center, 2400 Pratt St, Durham, NC 27705, USA.
Filippo Crea, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Gemelli, IRCCS, Università Cattolica del Sacro Cuore, Largo Agostino Gemelli 1, Roma 00168, Italy.
Stephen E Fremes, Division of Cardiac Surgery, Department of Surgery, Schulich Heart Centre, Sunnybrook Health Sciences Centre, University of Toronto, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada.
Sripal Bangalore, Division of Cardiology, NYU Langone Health, 27 W 86th St, New York, NY 10024, USA.
Funding
The authors report no specific funding related to this article.
Conflict of interest: Dr D.L.B. discloses the following relationships—Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, and Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Dr J.H.A. discloses the following relationships—Research support through Duke University from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CryoLife, CSL Behring, Ferring, GlaxoSmithKline, US Food and Drug Administration, US National Institutes of Health, and XaTek and personal consulting fees or honoraria from AbbVie, Bayer, Bristol-Myers Squibb, CryoLife, Inositec, Novo Nordisk, Portola, Quantum Genomics, the VA Cooperative Studies Program, and Zafgen. Dr S.B. discloses the following relationships—Research support from NHLBI, Abbott Vascular, REATA. Advisory board—Abbott Vascular, Biotronik, Pfizer, Amgen, REATA, Meril, SMT. Dr S.S. discloses research grant from Vascular Graft Solutions, honoraria from Vascular Graft Solutions, Somahlution. All other authors have nothing to disclose.
References
- 1. Gaudino M, Antoniades C, Benedetto U, Deb S, Di Franco A, Di Giammarco G, Fremes S, Glineur D, Grau J, He G-W, Marinelli D, Ohmes LB, Patrono C, Puskas J, Tranbaugh R, Girardi LN, Taggart DP, Ruel M, Bakaeen FG, ATLANTIC (Arterial Grafting International Consortium) Alliance. Mechanisms, consequences, and prevention of coronary graft failure. Circulation 2017;136:1749–1764. [DOI] [PubMed] [Google Scholar]
- 2. Gaudino M, Benedetto U, Fremes SE, Hare DL, Hayward P, Moat N, Moscarelli M, Di Franco A, Nasso G, Peric M, Petrovic I, Collins P, Webb CM, Puskas JD, Speziale G, Yoo KJ, Girardi LN, Taggart DP, RADIAL Investigators. Angiographic outcome of coronary artery bypass grafts: the radial artery database international alliance. Ann Thorac Surg 2020;109:688–694. [DOI] [PubMed] [Google Scholar]
- 3. Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W, VA Cooperative Study Group #207/297/364. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149–2156. [DOI] [PubMed] [Google Scholar]
- 4. Buxton BF, Hayward PA, Raman J, Moten SC, Rosalion A, Gordon I, Seevanayagam S, Matalanis G, Benedetto U, Gaudino M, Hare DL, Gaer J, Negri J, Komeda M, Bellomo R, Doolan L, McNicol L, Brennan J, Chan R, Clark D, Dick R, Dortimer A, Ecclestone D, Farouque O, Fernando D, Horrigan M, Jackson A, Oliver L, Mehta N, Nadurata V, Nadarajah N, Proimos G, Rowe M, Sia B, Webb C, Anaveker N, Barlis P, Calafiore P, Chan B, Cotroneo J, Johns J, Jones E, Kertes P, O’Donnell D, Sylviris S, Tonkin A, Fabini R, Kearney L, Lim R, Molan M, Smith G, Wellman C, Eng J, Hameed I, Shaw M, Gerbo S, RAPCO Investigators. Long-term results of the RAPCO trials. Circulation 2020;142:1330–1338. [DOI] [PubMed] [Google Scholar]
- 5. Ruiter MS, Pesce M. Mechanotransduction in coronary vein graft disease. Front Cardiovasc Med 2018;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Storey RF. Exploring mechanisms of graft occlusion toward improved outcomes in coronary artery bypass graft surgery. J Am Coll Cardiol 2011;57:1078–1080. [DOI] [PubMed] [Google Scholar]
- 7. Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg 2013;257:824–833. [DOI] [PubMed] [Google Scholar]
- 8. Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016;13:79–98. [DOI] [PubMed] [Google Scholar]
- 9. Deb S, Singh SK, Moussa F, Tsubota H, Une D, Kiss A, Tomlinson G, Afshar M, Sless R, Cohen EA, Radhakrishnan S, Dubbin J, Schwartz L, Fremes SE, Radial Artery Patency Study Investigators. The long-term impact of diabetes on graft patency after coronary artery bypass grafting surgery: a substudy of the multicenter Radial Artery Patency Study. J Thorac Cardiovasc Surg 2014;148:1246–1253; discussion 1253. [DOI] [PubMed] [Google Scholar]
- 10. Agarwal N, Mahmoud AN, Patel NK, Jain A, Garg J, Mojadidi MK, Agrawal S, Qamar A, Golwala H, Gupta T, Bhatia N, Anderson RD, Bhatt DL. Meta-analysis of aspirin versus dual antiplatelet therapy following coronary artery bypass grafting. Am J Cardiol 2018;121:32–40. [DOI] [PubMed] [Google Scholar]
- 11.Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med 1997;336:153–162. [DOI] [PubMed] [Google Scholar]
- 12. Shah AA, Haynes C, Craig DM, Sebek J, Grass E, Abramson K, Hauser E, Gregory SG, Kraus WE, Smith PK, Shah SH. Genetic variants associated with vein graft stenosis after coronary artery bypass grafting. Heart Surg Forum 2015;18:E1–E5. [DOI] [PubMed] [Google Scholar]
- 13. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 14. Hamon M, Lepage O, Malagutti P, Riddell JW, Morello R, Agostini D, Hamon M. Diagnostic performance of 16- and 64-section spiral CT for coronary artery bypass graft assessment: meta-analysis. Radiology 2008;247:679–686. [DOI] [PubMed] [Google Scholar]
- 15. Murphy GJ, Angelini GD. Insights into the pathogenesis of vein graft disease: lessons from intravascular ultrasound. Cardiovasc Ultrasound 2004;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616–626. [DOI] [PubMed] [Google Scholar]
- 17. Harskamp RE, Williams JB, Hill RC, de Winter RJ, Alexander JH, Lopes RD. Saphenous vein graft failure and clinical outcomes: toward a surrogate end point in patients following coronary artery bypass surgery? Am Heart J 2013;165:639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buxton BF, Durairaj M, Hare DL, Gordon I, Moten S, Orford V, Seevanayagam S. Do angiographic results from symptom-directed studies reflect true graft patency? Ann Thorac Surg 2005;80:896–900; discussion 900–901. [DOI] [PubMed] [Google Scholar]
- 19. Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT, PREVENT IV Investigators. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005;294:2446–2454. [DOI] [PubMed] [Google Scholar]
- 20. Deb S, Singh SK, de Souza D, Chu MWA, Whitlock R, Meyer SR, Verma S, Jeppsson A, Al-Saleh A, Brady K, Rao-Melacini P, Belley-Cote EP, Tam DY, Devereaux PJ, Novick RJ, Fremes SE, SUPERIOR SVG Study Investigators. SUPERIOR SVG: no touch saphenous harvesting to improve patency following coronary bypass grafting (a multi-Centre randomized control trial, NCT01047449). J Cardiothorac Surg 2019;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farooq V, Serruys PW, Garcia-Garcia HM, Zhang Y, Bourantas CV, Holmes DR, Mack M, Feldman T, Morice MC, Ståhle E, James S, Colombo A, Diletti R, Papafaklis MI, de Vries T, Morel MA, van Es GA, Mohr FW, Dawkins KD, Kappetein AP, Sianos G, Boersma E. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol 2013;61:282–294. [DOI] [PubMed] [Google Scholar]
- 22. Tonino PAL, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, MacCarthy PA, van't Veer M, Pijls NHJ. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–2821. [DOI] [PubMed] [Google Scholar]
- 23. Ferguson TB, Chen C, Babb JD, Efird JT, Daggubati R, Cahill JM. Fractional flow reserve-guided coronary artery bypass grafting: can intraoperative physiologic imaging guide decision making? J Thorac Cardiovasc Surg 2013;146:824–835.e1. [DOI] [PubMed] [Google Scholar]
- 24. Glineur D, Grau JB, Etienne P-Y, Benedetto U, Fortier JH, Papadatos S, Laruelle C, Pieters D, El Khoury E, Blouard P, Timmermans P, Ruel M, Chong A-Y, So D, Chan V, Rubens F, Gaudino MF. Impact of preoperative fractional flow reserve on arterial bypass graft anastomotic function: the IMPAG trial. Eur Heart J 2019;40:2421–2428. [DOI] [PubMed] [Google Scholar]
- 25. Spadaccio C, Glineur D, Barbato E, Di Franco A, Oldroyd KG, Biondi-Zoccai G, Crea F, Fremes SE, Angiolillo DJ, Gaudino M. Fractional flow reserve-based coronary artery bypass surgery: current evidence and future directions. JACC Cardiovasc Interv 2020;13:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morice M-C, Feldman TEE, Mack MJ, Ståhle E, Holmes DR, Colombo A, Morel M-A, van den Brand M, Serruys PW, Mohr F, Carrié D, Fournial G, James S, Leadley K, Dawkins KD, Kappetein AP. Angiographic outcomes following stenting or coronary artery bypass surgery of the left main coronary artery: fifteen-month outcomes from the synergy between PCI with TAXUS express and cardiac surgery left main angiographic substudy (SYNTAX-LE MANS). EuroIntervention 2011;7:670–679. [DOI] [PubMed] [Google Scholar]
- 27. Robert EW, Guthaner DF, Wexler L, Alderman EL. Six-year clinical and angiographic follow-up of patients with previously documented complete revascularization. Circulation 1978;58:I194–I199. [PubMed] [Google Scholar]
- 28. Bourassa MG, Campeau L, Lespérance J, Grondin CM. Changes in grafts and coronary arteries after saphenous vein aortocoronary bypass surgery: results at repeat angiography. Circulation 1982;65:90–97. [DOI] [PubMed] [Google Scholar]
- 29. Laird-Meeter K, ten Katen HJ, Brower RW, van den Brand MJ, Serruys PW, Haalebos MM, Bos E, Hugenholtz PG. Angina pectoris, one to 10 years after aortocoronary bypass surgery. Eur Heart J 1983;4:678–686. [DOI] [PubMed] [Google Scholar]
- 30. Brindis RG, Brundage BH, Ullyot DJ, McKay CW, Lipton MJ, Turley K. Graft patency in patients with coronary artery bypass operation complicated by perioperative myocardial infarction. J Am Coll Cardiol 1984;3:55–62. [DOI] [PubMed] [Google Scholar]
- 31. Lytle BW, Loop FD, Taylor PC, Simpfendorfer C, Kramer JR, Ratliff NB, Goormastic M, Cosgrove DM, Schnauffer MJ. Vein graft disease: the clinical impact of stenoses in saphenous vein bypass grafts to coronary arteries. J Thorac Cardiovasc Surg 1992;103:831–840. [PubMed] [Google Scholar]
- 32. Huikuri HV, Yli-Mäyry S, Airaksinen KE, Ikäheimo MJ, Linnaluoto MK, Takkunen JT. Clinical and angiographic prediction of cardiac death after coronary artery bypass graft surgery. Br Heart J 1992;67:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knatterud GL, White C, Geller NL, Campeau L, Forman SA, Domanski M, Forrester JS, Gobel FL, Herd JA, Hickey A, Hoogwerf BJ, Hunninghake DB, Terrin ML, Rosenberg Y. Angiographic changes in saphenous vein grafts are predictors of clinical outcomes. Am Heart J 2003;145:262–269. [DOI] [PubMed] [Google Scholar]
- 34. Halabi AR, Alexander JH, Shaw LK, Lorenz TJ, Liao L, Kong DF, Milano CA, Harrington RA, Smith PK. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol 2005;96:1254–1259. [DOI] [PubMed] [Google Scholar]
- 35. Lopes RD, Mehta RH, Hafley GE, Williams JB, Mack MJ, Peterson ED, Allen KB, Harrington RA, Gibson CM, Califf RM, Kouchoukos NT, Ferguson TB, Alexander JH, Project of Ex Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) Investigators Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation 2012;125:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shavadia J, Norris CM, Graham MM, Verma S, Ali I, Bainey KR. Symptomatic graft failure and impact on clinical outcome after coronary artery bypass grafting surgery: results from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease registry. Am Heart J 2015;169:833–840. [DOI] [PubMed] [Google Scholar]
- 37. Harskamp RE, Alexander JH, Ferguson TB, Hager R, Mack MJ, Englum B, Wojdyla D, Schulte PJ, Kouchoukos NT, de Winter RJ, Gibson CM, Peterson ED, Harrington RA, Smith PK, Lopes RD. Frequency and predictors of internal mammary artery graft failure and subsequent clinical outcomes: insights from the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT) IV trial. Circulation 2016;133:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamasaki M, Deb S, Tsubota H, Moussa F, Kiss A, Cohen EA, Radhakrishnan S, Dubbin J, Ko D, Schwartz L, Fremes SE, Radial Artery Patency Study Investigators. Comparison of radial artery and saphenous vein graft stenosis more than 5 years after coronary artery bypass grafting. Ann Thorac Surg 2016;102:712–719. [DOI] [PubMed] [Google Scholar]
- 39. Winer HE, Glassman E, Spencer FC. Mechanism of relief of angina after coronary bypass surgery: an angiographic study. Am J Cardiol 1979;44:202–208. [DOI] [PubMed] [Google Scholar]
- 40. Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, Golding LAR, Gill CC, Taylor PC, Sheldon WC, Proudfit WL. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1–6. [DOI] [PubMed] [Google Scholar]
- 41. Zeff RH, Kongtahworn C, Iannone LA, Gordon DF, Brown TM, Phillips SJ, Skinner JR, Spector M. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg 1988;45:533–536. [DOI] [PubMed] [Google Scholar]
- 42. Gaudino M, Benedetto U, Fremes S, Biondi-Zoccai G, Sedrakyan A, Puskas JD, Angelini GD, Buxton B, Frati G, Hare DL, Hayward P, Nasso G, Moat N, Peric M, Yoo KJ, Speziale G, Girardi LN, Taggart DP, RADIAL Investigators. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med 2018;378:2069–2077. [DOI] [PubMed] [Google Scholar]
- 43. Korpilahti K, Engblom E, Hämäläinen H, Syvänne M, Hietanen E, Arstila M, Puukka P, Rönnemaa T. Significance of graft occlusion and coronary atherosclerosis 5 years after coronary artery bypass grafting. A quantitative angiographic study with serial exercise testing. J Intern Med 1999;245:545–552. [DOI] [PubMed] [Google Scholar]
- 44. Blankenhorn DH, Nessim SA, Johnson RL, Sanmarco ME, Azen SP, Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA 1987;257:3233–3240. [PubMed] [Google Scholar]
- 45. Solo K, Lavi S, Kabali C, Levine GN, Kulik A, John-Baptiste AA, Fremes SE, Martin J, Eikelboom JW, Ruel M, Huitema AA, Choudhury T, Bhatt DL, Tzemos N, Mamas MA, Bagur R. Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. BMJ 2019;367:l5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Achuff SC, Griffith LS, Conti CR, Humphries JO, Brawley RK, Gott VL, Ross RS. The “angina-producing” myocardial segment: an approach to the interpretation of results of coronary bypass surgery. Am J Cardiol 1975;36:723–733. [DOI] [PubMed] [Google Scholar]
- 47. Benchimol A, dos Santos A, Desser KB. Relief of angina pectoris in patients with occluded coronary bypass grafts. Am J Med 1976;60:339–343. [DOI] [PubMed] [Google Scholar]
- 48. Hoel B, Eie H, Semb G, Sivertssen E. Aortocoronary vein bypass in patients with angina pectoris. Acta Med Scand 2009;197:383–390. [DOI] [PubMed] [Google Scholar]
- 49. Hattler B, Carr BM, Messenger J, Spertus J, Ebrahimi R, Bishawi M, Quin JA, Almassi GH, Collins JF, Kozora E, Grover FL, Shroyer ALW. Clinical and angiographic predictors of patient-reported angina 1 year after coronary artery bypass graft surgery. Circ Cardiovasc Qual Outcomes 2019;12:e005119. [DOI] [PubMed] [Google Scholar]
- 50. Aintablian A, Hamby RI, Hoffman I, Weisz D, Voleti C, Wisoff BG. Significance of new Q waves after bypass grafting: correlations between graft patency, ventriculogram, and surgical venting technique. Am Heart J 1978;95:429–440. [DOI] [PubMed] [Google Scholar]
- 51. Biancari F, Anttila V, Dell’Aquila AM, Airaksinen JKE, Brascia D. Control angiography for perioperative myocardial Ischemia after coronary surgery: meta-analysis. J Cardiothorac Surg 2018;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoon SH, Kim YH, Yang DH, Roh JH, Lee EY, Lee PH, Sugiyama D, Chang M, Ahn JM, Choi WJ, Kang JW, Lim TH, Kim JB, Jung SH, Chung CH, Choo SJ, Lee JW, Kang SJ, Park DW, Lee SW, Lee CW, Park SW, Park SJ. Risk of new native-vessel occlusion after coronary artery bypass grafting. Am J Cardiol 2017;119:7–13. [DOI] [PubMed] [Google Scholar]
- 53. Kroncke GM, Kosolcharoen P, Clayman JA, Peduzzi PN, Detre K, Takaro T. Five-year changes in coronary arteries of medical and surgical patients of the Veterans Administration Randomized Study of Bypass Surgery. Circulation 1988;78:I144–I150. [PubMed] [Google Scholar]
- 54. Cashin WL, Sanmarco ME, Nessim SA, Blankenhorn DH. Accelerated progression of atherosclerosis in coronary vessels with minimal lesions that are bypassed. N Engl J Med 1984;311:824–828. [DOI] [PubMed] [Google Scholar]
- 55. Dimitrova KR, Hoffman DM, Geller CM, Dincheva G, Ko W, Tranbaugh RF. Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg 2012;94:475–481. [DOI] [PubMed] [Google Scholar]
- 56. Zhang M, Guddeti RR, Matsuzawa Y, Sara JDS, Kwon TG, Liu Z, Sun T, Lee SJ, Lennon RJ, Bell MR, Schaff HV, Daly RC, Lerman LO, Lerman A, Locker C. Left internal mammary artery versus coronary stents: impact on downstream coronary stenoses and conduit patency. J Am Heart Assoc 2016;5:e003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alderman EL, Corley SD, Fisher LD, Chaitman BR, Faxon DP, Foster ED, Killip T, Sosa JA, Bourassa MG. Five-year angiographic follow-up of factors associated with progression of coronary artery disease in the Coronary Artery Surgery Study (CASS). CASS Participating Investigators and Staff. J Am Coll Cardiol 1993;22:1141–1154. [DOI] [PubMed] [Google Scholar]
- 58. Zhu YY, Hayward PAR, Hare DL, Reid C, Stewart AG, Buxton BF. Effect of lipid exposure on graft patency and clinical outcomes: arteries and veins are different. Eur J Cardiothorac Surg 2014;45:323–328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.