Abstract
Developing effective digital interventions to help patients form healthy habits is a challenging goal. IDEAS is a step-by-step framework that allows developers to draw ideas from intended users and behavioral theories, and ideate implementation strategies for them, followed by rapid prototype development. Based on our long experience with developing generic knowledge-based clinical decision support systems (CDSS) and integrating them with electronic health records (EHR) to deliver patient-specific advice, we observed a challenge that IDEAS is not addressing: the semantic detailing of the clinical knowledge behind the digital intervention and relevant patient data that could be used to personalize the digital intervention. To close the gap, we augmented two steps of IDEAS with an ontology that structures the target behavior as classes, derived from HL7 Fast Healthcare Interoperability Resources standard. We exemplify the augmented IDEAS with a case study taken from the Horizon 2020 CAPABLE project, that uses Fogg's Tiny Habits behavioral model to improve the sleep of cancer patients via Tai Chi.
Keywords: Clinical decision support; Mobile Health; Knowledge Representation and Information Modeling; Controlled Terminologies Ontologies, and Vocabularies; User-centered Design Methods; behavior change; Fogg Behavioral Model
1. Introduction
People want to change their behaviors to improve their quality of life and make healthier choices. The most frequent behavior change areas [1] are smoking cessation, sleep quality improvement, weight loss, diet, physical activity, treatment adherence (i.e., adherence to medical recommendations including both taking medication and attending appointments), especially for chronic disease management. Digital interventions delivered through mobile technology can have great potential to facilitate such health behavior changes, as they provide improvements in efficacy, cost-effectiveness, safety, and scalability. Moreover, the literature highlights many successful digital interventions [2] developed for different types of behavioral changes such as Vegethon [3] for nutritional intervention, Happy Ending [4] for smoking cessation, SMART [5] for weight loss goal, and Headspace [6] for mindfulness. These systems cited above all utilize behavioral health techniques [7]. However, we have not encountered any ontologies that allow to detail the properties of the behavioral interventions that make them actionable in a general system.
To support the development of effective digital interventions for behavioral change, Mummah et al. [8] developed a framework and method called IDEAS (Integrate, DEsign, Assess, and Share). This framework uses a step-by-step approach to guide the development process using behavioral theory, design thinking, user-centered design, rigorous evaluation, and dissemination. While we find the IDEAS framework to be extremely constructive and useful, we believe it lacks formal and detailed structuring of digital interventions. In a previous study [9], we extended the IDEAS abstract concepts into concrete backend architectural components and graphical user-interface designs. In this paper, we propose an extension of IDEAS using an ontology to provide a conceptual framework for the definition of the behavior intervention. The behavioral theories exploited by the digital intervention and the clinical evidence for their success guide the structuring of the knowledge base. To structure the ontology, we rely on HL7 standards, and in particular on HL7's Fast Healthcare Interoperability Resources (FHIR) [10] standard. Defining a FHIR-based ontology allows standardizing the knowledge related to digital interventions for behavioural changes, which has not been done so far. Furthermore, this represents a step towards making the intervention actionable as an integrated part of a clinical decision support system (CDSS).
In this paper, we introduce the general methodology that we developed for the construction of such ontology, which is reusable in different application domains and for different target behaviors. We demonstrate our method through a case study taken from the Horizon 2020 CAncer PAtient Better Life Experience (CAPABLE) project (https://capable-project.eu/), where we are using Fogg's Tiny Habits behavioral model [11] to improve the sleep of cancer patients.
2. Related work
The IDEAS framework
Mummah et al. [8] presented a ten-step guide to the development of effective digital health interventions that provides a disciplined way to incrementally translate behavioral theories into highly relevant and practical interventions. The ten steps are organized into a four-phases process as follows: Integrate phase, including (1) empathize with target users, (2) specify target behavior, (3) ground in behavioral theory; DEsign phase, including (4) ideate implementation strategies, (5) prototype potential products, (6) gather user feedback, (7) build a minimum viable product; Assess phase, including (8) pilot test to assess potential efficacy and usability, and (9) evaluation of the efficacy in an RCT; and Share phase, to (10) share intervention and findings.
Fogg's Tiny Habits behavioral model
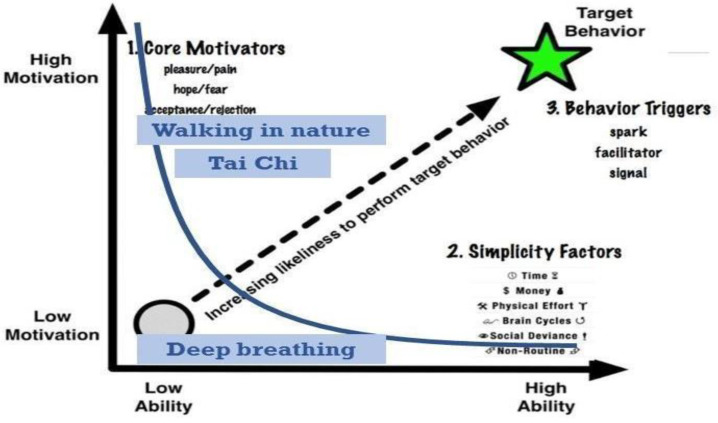
Fogg Behavioral Model (FBM) [11] is especially suitable to help patients turn interventions into habits. The model includes three main concepts: motivation, ability, and triggers. If all are present, they allow the behavioral change to occur and become a habit. Fig. 1 shows the core concepts of FBM. The vertical axis represents the motivation, which is driven by three different core motivators (pleasure/pain, hope/fear, and acceptance/rejection). The horizontal axis presents the ability of the subject to perform the behavioral task. Ability is affected by six different elements, called simplicity factors (time, money, physical effort, brain cycles, social deviance, and non-routine). The target behaviour has a trigger that prompts the subject to perform the behavioral task. Triggers can be a spark, a facilitator, or a signal.
Fig. 1.
Fogg's Behavioral Model with examples of the capsules for a specific subject, shown in blue. Adapted from [11].
This representation suggests, as the model asserts, that a person is able to achieve a target behavior if s/he has high motivation, high ability, and an effective trigger at the same instant; this places the person to the top-right of the curve, which is the actionable part where habits are formed. Note that in Fig. 1, three virtual interventions are shown as blue squares. Deep Breathing is not actionable as it is below the curve. In order to allow a person to adopt a behavior, we can increase her motivation or increase her ability by making the needed behavior smaller and easier to do.
Patient data models facilitating personalized decision support
In a previous CDSS that we developed, MobiGuide [12], we defined a patient model that included the patient's clinical data, patient-reported symptoms, personal preferences, psychosocial context, and personal events. The model is both dynamic, as it reacts to current data from real-time monitoring of patient's reporting and sensors tracking, and it is also highly adaptive to the personal preferences and contexts of the individual patient. The adaptive part of the model allowed us to suggest digital interventions that best fit with the patient's characteristics. The model complied with HL7's Virtual Medical Record.
Since that time, HL7 has released a more robust standard called Fast Healthcare Interoperability Resources (FHIR) [10]. It leverages existing logical and theoretical models to provide a consistent, easy to implement, and rigorous mechanism for exchanging data between healthcare applications. To achieve this goal, FHIR uses generic Resources (i.e., Patient, Observation), that define the properties of all exchangeable information.
3. Methods
We augmented two steps of the IDEAS framework with an ontology structuring digital interventions for behavioral change. An ontology is an explicit and formal specification of a shared conceptualization representing a consensual and shared knowledge of an abstract model of a world's domain or phenomenon [13]. By defining classes in the ontology, we can specify the main concepts related to digital non-pharmacological evidence-based interventions, which we call "virtual capsules". In addition, we also represent a patient model useful for creating personalized interventions.
To define the structure of the classes in the ontology, we leverage the SNOMED-CT vocabulary and HL7 FHIR resources (a) Patient, (b) Medication_Request, (c) Medication, and (d) Dosage. The ontology provides a way to model a structured conceptual framework representing knowledge on specific concepts and the relations among them. This provides an instrument that allows more expressiveness that simply extending "isolated" FHIR resources. Moreover, using an ontology would allow extending and using the model with standards other than HL7 FHIR. Our framework is generalizable because it in principle allows representing any kind of non-pharmacological intervention. It is in fact possible to add other types of capsules targeting a wide variety of behaviours and goals.
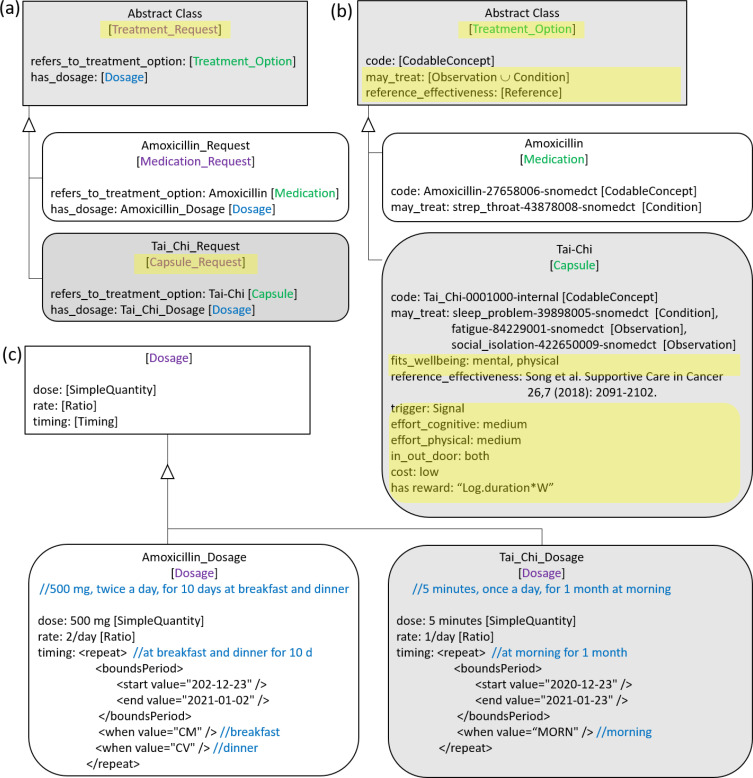
To conform to the Basic Formal Ontology [14], Patient is an Independent_Continuant, Medication is a Process_Profile, Medication is an Object, and Dosage is a Genertically_Dependent_Continuant. Medication_Request models an order for the medication and the instructions for administration of the medication to a patient, including dosage which also includes timing. Although, as observed in [15], FHIR does not address non-pharmacological behavioral interventions, we extend FHIR by using the analogy between pharmacological treatments and virtual Capsules representing behavioral interventions. These two types of interventions share several characteristics, except for the fact that pharmacological interventions use drugs, whereas the interventions in the capsules are lifestyle changes related to cognitive behavioral therapies or physical activity. Therefore, in our ontology, both Medication and virtual Capsule are modeled as subclasses of a new, more general class, called Treatment_Option (Fig 2b). Similarly, Treatment_Request (Fig 2a) is a new class that generalizes Medication_Request and the new analogous subclass Capsule_Request. As in FHIR, Treatment_Request includes dosage information. Fig 2c shows instances of Dosage for a Medication_Request and for a Capsule_Request.
Fig. 2.
The analogy between Medication_Request and (virtual) Capsule_Request for behavioral interventions. Our additions to FHIR resources are highlighted in yellow.
Similar to the FHIR Medication resource, Treatment_Option has a codable concept that specifies the preferred name and controlled clinical vocabulary code (e.g., SNOMED-CT code) for the medications or the digital behavioral intervention (e.g., Mindfulness, Tai Chi), for which internal codes are used.
In addition, the Treatment_Option class structures the interventions by providing a goal-based and evidence-based view. The goal of Treatment Option was inspired by the Goal structure for CDSSs developed by Kogan et al. [16], which uses the relationship may_treat of the National Drug File Reference Terminology (NDFRT) [17]. Hence Treatment option has the property may_treat, whose domain is FHIR Observation (e.g., "may treat sleep problems" or "may treat fatigue"). For the Capsule subclass, goals are extended to wellbeing dimensions by adding the property fits_wellbeing, whose domain is Wellbeing_Dimension (i.e., mental, social, physical, spiritual, functioning, and global wellbeing [18]). The evidence-based view provides Treatment_Option with a property to cite evidence that the proposed intervention is effective for the goal (reference_effectiveness).
The Capsule class include additional properties corresponding to the theoretical behavioral model behind the intervention. The interventions that have been developed for the CAPABLE project are all based on the FBM [9]. To take this into account, the Capsule class has a set of properties that reflect factors related to trigger, ability, and motivation. The trigger (prompt) property can represent a signal, spark or facilitator. The two properties called effort_cognitive and effort_physical represent the FBM ability factors related to the mental and physical effort required for the specific behaviour. The two properties cost and in_out_doors are ability factors according to FBM. The FBM motivation factor is modeled through a reward function (has_reward) that assigns a score to the patient at the end of the execution of the practice.
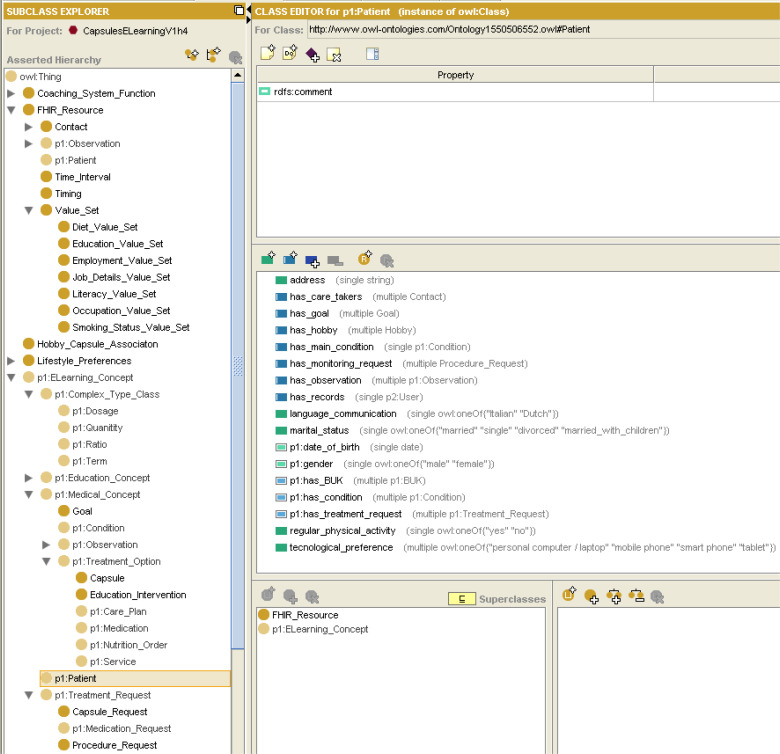
Finally, the Patient class includes properties of the Patient FHIR resource, including name, gender, date of birth, marital status, care takers, and communication language. Similar to the patient model developed in [12], the Patient class also includes the main diagnosis and comorbidities or the patient, and the prescribed Treatment_Requests, which connects the patient to the patient-specific digital interventions and medications (Capsule_Request and Medication_Request). To allow goal-based reasoning [16] that is used in CAPABLE CDSS, we also add the clinical goals of the patient. As in [12], the Patient class also contains the observations related to the patient. Similar to the patient preferences used in [12], we represent the patient's ambiance preferences, musical preferences, technological preferences, and physical activity preferences, which allow personalization of recommendations.
We developed the ontology via the Protégé knowledge modeling tool (protégé.standord.edu) version 3.4.8 using Web Ontology Language (OWL) version 1. Examples for instances of our extensions, for an intervention based on FBM [11], are presented in Section 4.
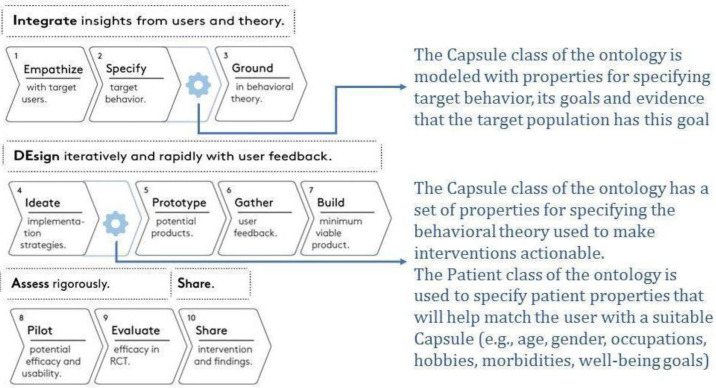
The presented ontology would fit well into two steps of the IDEAS framework (Fig. 3). The "specify target behavior" step of the "Integrate" phase is focused on making the target behavior highly specific and actionable, and providing the evidence for its potential impact. The goal of our ontology is to make the structure of the intervention explicit. In more detail, some of the properties of the Capsule class (may_treat and fits_wellbeing, for the goal-based view, reference_effectiveness for the evidence_based view) and the timing property of the Dosage class are specifically devoted to indicate the target of the intervention and to ground its validity on scientific evidence.
The second step of the IDEAS framework that would benefit from the introduction of the presented ontology is the "ideate" step of the Design phase. This step is focused on the implementation strategies. To this end, the Capsule class is provided with a set of properties to explicitly refer to the behavioural model which is used to put the intervention into place (e.g., FBM). The Capsule_Request class is specifically aimed at modeling the implementation of the intervention on the specific user, which is in turn modelled using the Patient class. The properties designed in the Patient class make it possible to propose and recommend the user the digital interventions that best fit with his intrinsic characteristics and preferences.
As shown in Fig. 3, the presented ontology extends the IDEAS framework in two specific steps. To get to the final structure of the ontology, though, we also slightly modified two other phases: Empathize and Gather. In IDEAS the users that are involved in collecting insights and requirements are patients, while we have relied on multiple stakeholders, including clinicians, psychologists, nutritionists, clinical and informatics researchers, and software developers. Each of them brought input to the requirement collection, as related to the virtual capsules (behavioral interventions).
Fig. 3.
The IDEAS framework [8], extended by our method. Changes are in blue.
4. Results in the CAPABLE Case Study
Most cancer patients are managed at home, facing long-term treatments that make the disease comparable to a chronic condition [19]. Patients are expected to assume a more significant role in managing their follow-up care. They must have the ability to control the symptoms and the consequences of living with a chronic condition, including treatment, physical, social and lifestyle behavioral changes [20]. To improve patients' well-being [18] and to help them comply with behavioral interventions known to improve mental and physical health, we hypothesize that mobile technology can potentially support. Thus, we had ideated virtual capsules, which serve as the knowledge base of a CDSS patient coaching system.
These digital interventions were developed as part of a European project, part of the Horizon 2020 European program for research and innovation, called CAPABLE (CAncer PAtients Better Life Experience), that aims at building a CDSS to support cancer patients and their care providers during the home management of the disease, using Artificial Intelligence and Big Data potentialities.
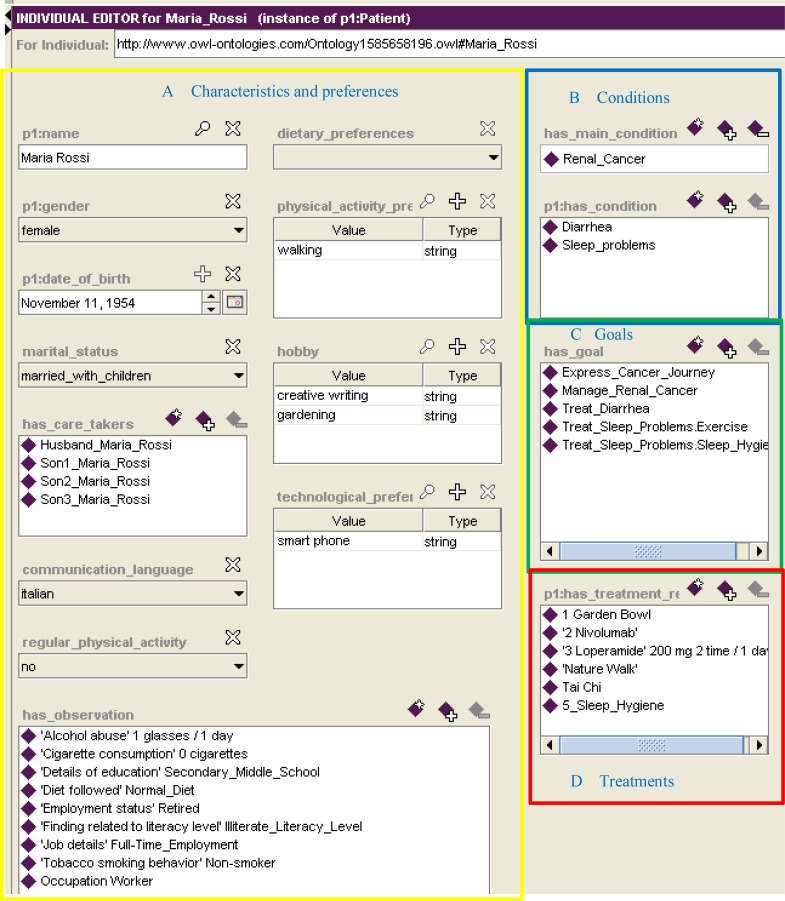
In this paper we use CAPABLE as a case study for the design and the implementation of digital interventions using the ontology presented in Section 3. In our case study we profile a patient called, Maria Rossi, a hypothetical Italian female kidney cancer patient aged 66. Maria is modelled as an individual of the Patient class (Fig 4), and she has properties related to personal information (e.g., name, gender, marital_status, care takers, communication language) and life-style preferences (e.g., diet, physical activity, hobbies –following Wikipedia's list of hobbies [21], and technological preferences (i.e., PC/laptop, mobile phone, smart phone, tablet) (Fig 4A), clinical conditions (e.g., main cancer condition and comorbidities of diarrhea and sleep problems) (Fig 4B), goals that the patient wants to achieve (Fig 4C), and prescribed treatments for these goals, including medications and virtual capsules (Fig 4D).
Fig. 4.
Maria Rossi, an individual of the Patient class of the ontology
This profiling helps us to better associate users with personalized digital interventions. In fact, thanks to Maria’s preferences, it is possible to identify some capsules suitable for her, and others that might be inappropriate. As it is possible to see from the regular_physical_activity property value in Figure 4A, Maria does not practice regular physical activity. Tai Chi, which is a short physical and mental practice, could help her increase her level of physicality without too much effort. Moreover, since Maria likes to walk (physical_activity_preference property), a digital intervention such as the daily walk in nature can also fit well with Maria's characteristics. Instead, a static capsule like Deep Breathing, a relaxation technique, is considered unsuitable for Maria, as it does not match her interests.
Fig. 1 shows how the capsules identified as suitable for Maria appear in the actionable part of the Fogg Behavioral Model, as the user has the necessary characteristics to have sufficient ability and motivation to carry out the interventions that are to the right of the curve. Capsules such as Deep Breathing appear below this actionable part, because Maria's motivation for them is low; to make interventions more appealing to the patient it is necessary to modify them according to the user's preferences and needs.
As previously mentioned, the Patient class has a property that connects it to individuals of the class Treatment_Request (has_treatment_request – Figure 4D). In the specific case of a behavioural intervention, this property allows us to link the Patient class with the Capsule_Request class, that models the knowledge related to digital intervention prescribed for a single patient.
One of the treatments that is requested for Maria is the Tai Chi (Fig 4D), which is modeled as an individual of the Capsule_Request class, as shown in Figure 2. In particular, Maria's Capsule Request (Fig 2a) points to the Tai Chi Capsule (Fig 2b). Instances of the Capsule contain all the generic characteristics of the Tai Chi intervention, not strictly related to the user who has to practice it (Fig 2b). The dosage and timing properties of the recommended Treatment_Request (Fig 2a) are represented through an individual of the class Dosage (Fig 2c). The dose, rate, and timing can be used to personalize the intervention to the patient's ability. For example, for some patients, 30 minutes of Tai Chi could be too much, but they could form a habit of practicing Tai Chi 5 minutes a day (dose) every day (rate) for one month, in the morning (timing).
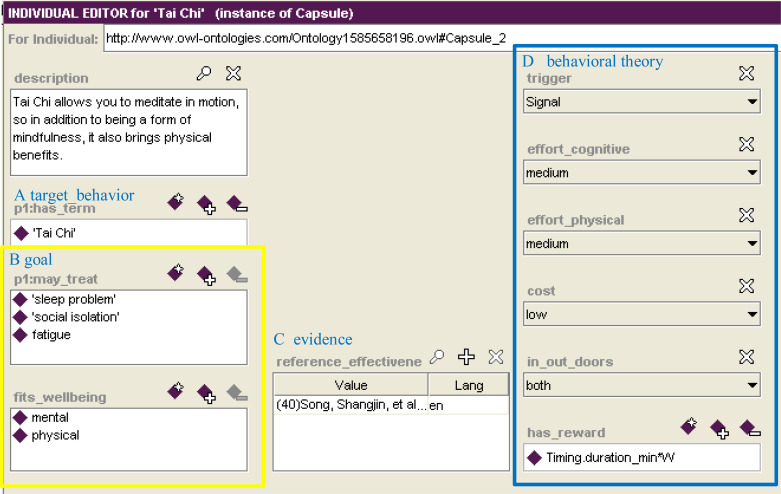
The Tai-Chi Capsule instance for Maria is shown in Figure 5. It specifies the target behavior (Fig 5A) and the goals that the intervention aims to achieve (Fig 5B), through the properties may_treat and fits_wellbeing. These properties model that this capsule aims to improve sleep problems and fatigue thus improving physical and emotional well-being. Since every digital intervention developed in our work is based on scientific evidence, the Capsule class contains the reference_effectiveness property (in Figure 5.C), which points at the benefits that the capsule can carry out to solve this issues (in this case the ameliorative effects that Tai Chi can bring in cancer patients).
Fig. 5.
Tai Chi individual of Capsule class
Part D of Fig. 5 relates the Capsule to the FBM [11]. The trigger chosen for Tai Chi belongs to the Signal type, as notifications are sent to Maria, through the application, that remind her to perform the practice. The effort_cognitive and effort_physical related to the Tai Chi capsule are considered of medium intensity, as Tai Chi requires both mindfulness and body movement. The cost is low, as CAPABLE offers Maria some YouTube videos to perform the practice. Tai Chi may be practiced in-or out-doors. The FBM motivation factor is modeled through a reward function (has_reward) that assigns a score to Maria at the end of the execution of the practice, depending on the duration of the exercise.
Figure 6 presents the taxonomy of classes in our ontology and the properties of the Patient class, exemplified by the Patient individual of Figure 4.
Figure 6.
The taxonomy of classes in our ontology and the properties of the Patient class
4. Discussion
In this paper we presented the extension of the IDEAS framework with a structured ontology that allows developers of digital behavioral change interventions to address in a systematic and detailed way the specification of the behavioral change intervention (the Capsule), its dosage and timing (Treatment_Request), and the properties of the Patient that could be used to personalize the Capsule_Request to the patient's characteristics and preferences. By basing our ontology on FHIR resources, we allow modelers and developers of digital interventions a standard way to specify CDSS knowledge in a way that is interoperable with electronic health records. We have used the modified IDEAS framework and the novel ontology that we had developed in our ongoing development of the CAPABLE prototype, which is now in its second iteration and will be used by patients and clinicians in 2023. Its preliminary evaluation has been done by over ten additional members of the CAPABLE project, including software developers, clinicians and patients.
The novel Capsule class structures the potential delivery model of the digital intervention according to Fogg Behavioral Model [11]. As part of the CAPABLE project we aim to use machine learning models that would learn how to dynamically personalize the customizable capsules by modulating the duration of the Capsule_Request or its intensity level in order to meet the patient's "ability” factor of FBM. We have also started to develop methods for personalizing triggers by learning the user’s daily routine from sensors - when is she usually outside, when is she not moving enough, when is she sleeping, etc. This information could be used to predict the most suitable time for triggering (prompting) the reminder for the Capsule. We also started thinking about reward functions, as part of the motivation factor of FBM. Another part of the motivation is educating the patient about the benefits of adhering to the capsule recommendations (Capsule Request).
To make the capsules more actionable, future work will target quantifying the effort required to complete the capsule (physical and cognitive) as well as the ability and motivation of the patient. The effort required for the different capsules can be determined in two ways. The first is the available evidence on the effectiveness of the activity in promoting behavioral change (e.g., we know that Tai Chi is effective if performed every day for 5 minutes, which implies a medium physical effort). The second way to determine the effort is to rely on the knowledge of the expert (physician, psychologist, physiotherapist) who is defining the capsule.This work lays the foundation for a CAPABLE's virtual coaching system which will deliver personalized digital behavioral interventions to improve cancer patients’ wellbeing. Although our study is aimed at supporting the wellbeing of cancer patients, the behavioral change interventions that we offer could help all other chronic disease patients, whose physical health is often accompanied by mental distress. The mindfulness, nature-based, exercise-based, or positive psychology-based interventions that we offer are applicable to many other chronic disease patients. Furthermore, the work represents a step towards making digital interventions actionable as an integrated part of any knowledge-based CDSS. While we demonstrated our ideas using a particular case study, the ontology is generic and could be extended to support other behavioral models in addition to FBM.
Using the ontology would benefit developers and researchers of digital interventions beyond CAPABLE from the following aspects: (a) structuring their non-pharmacological intervention using a systematically-developed ontology leveraging on the available standards (FHIR, NDFRT, SNOMED-CT), (b) using the same ontology structure for different components of a complex CDSS in order to define the behavioural interventions but also to exchange information about its usage among system components, including the patient electronic health record, and (c) allowing a standard way for researchers to analyze data collected about the delivered interventions (both pharmacological and non-pharmacological) and their impact on the patient's health and wellbeing in a unified way. Beyond the contribution of this research toward representation of digital behavioral health interventions via an ontological standard-based information model, the integration into the IDEAS framework supports the entire life-cycle of eliciting requirements till implementing a mobile health app.
Acknowledgement
The work described in this article has been funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 875052 - CAPABLE (www.capable-project.eu).
Figures & Table
References
- 1.Riley William T, et al. “Health behavior models in the age of mobile interventions: are our theories up to the task?.”. Translational behavioral medicine 1.1. 2011:53–71. doi: 10.1007/s13142-011-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klasnja P, Pratt W. Healthcare in the pocket: mapping the space of mobile-phone health interventions. J Biomed Inform. 2012 Feb;45(1):184–98. doi: 10.1016/j.jbi.2011.08.017. doi: 10.1016/j.jbi.2011.08.017. Epub 2011 Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mummah SA, King AC, Gardner CD, Sutton S. Iterative development of Vegethon: A theory-based mobile app intervention to increase vegetable consumption. Int. J. Behav. Nutr. Phys. Act. 2016;13(1):1–12. doi: 10.1186/s12966-016-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brendryen H, Drozd F, Kraft P. A Digital Smoking Cessation Program Delivered Through Internet and Cell Phone Without Nicotine Replacement (Happy Ending): Randomized Controlled Trial. JMIR. 2008;10(5):e51. doi: 10.2196/jmir.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godino JG, Merchant G, Norman GJ, Donohue MC, Marshall SJ, Fowle JH, et al. Using social and mobile tools for weight loss in overweight and obese young adults (Project SMART): a 2 year, parallel-group, randomised, controlled trial. Lancet Diabetes Endocrinol. 2016;4(9):747–55. doi: 10.1016/S2213-8587(16)30105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Headspace. Headspace Mindfulness app. https://www.headspace.com/about-us. [Google Scholar]
- 7.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Heal Psychol. 2008;27(3):379–87. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 8.Mummah SA, Robinson TN, King AC, Gardner CD, Sutton S. IDEAS (Integrate, Design, Assess, and Share): A Framework and Toolkit of Strategies for the Development of More Effective Digital Interventions to Change Health Behavior. JMIR. 2016;18(12):e317. doi: 10.2196/jmir.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peleg M, Michalowski W, Wilk S, Parimbelli E, Bonaccio S, O’Sullivan D, et al. Ideating Mobile Health Behavioral Support for Compliance to Therapy for Patients with Chronic Disease: A Case Study of Atrial Fibrillation Management. J. Med. Syst. 2018;42:234–48. doi: 10.1007/s10916-018-1077-4. [DOI] [PubMed] [Google Scholar]
- 10.HL7. Fast Healthcare Interoperability Resources Release 3 Specification. 2017 https://www.hl7.org/fhir/overview.html. [Google Scholar]
- 11.Fogg BJ. Tiny Habits: The Small Changes That Change Everything. Houghton Mifflin Harcourt; 2020. [Google Scholar]
- 12.Peleg M, Shahar Y, Quaglini S, Fux A, García-Sáez G, Goldstein A, et al. MobiGuide: a personalized and patient-centric decision-support system and its evaluation in the atrial fibrillation and gestational diabetes domains. User Model. Useradapt. Interact. 2017;27(2):159–213. [Google Scholar]
- 13.Studer R, Benjamins R, Fensel D. Knowledge engineering: principles and methods. Data Knowl. Eng. 1998;25(1–2):161–97. [Google Scholar]
- 14.Grenon P, Smith B. SNAP and SPAN: Towards Dynamic Spatial Ontology. 2004;1(March):69–103. [Google Scholar]
- 15.Saripalle R. Integrating Physical Activity Data with Electronic Health Record. Proc. 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC) 2019:pages 21–29. ISBN: 978-989-758-353-7. [Google Scholar]
- 16.Kogan A, Peleg M, Tu SW, Allon R, Khaitov N, Hochberg I. Towards a goal-oriented methodology for clinical-guideline-based management recommendations for patients with multimorbidity: GoCom and its preliminary evaluation. J Biomed Inform. 2020 Dec;112:103587. doi: 10.1016/j.jbi.2020.103587. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Veterans Affairs. National Drug File - Reference Terminology (NDF-RTTM) Documentation. 2015 https://evs.nci.nih.gov/ftp1/NDF-RT/NDF-RTDocumentation.pdf. [Google Scholar]
- 18.Linton M-J, Dieppe P, Medina-Lara A. Review of 99 self-report measures for assessing well-being in adults: exploring dimensions of well-being and developments over time. BMJ Open. 2016 doi: 10.1136/bmjopen-2015-010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Cancer Society. Managing Cancer as a Chronic Illness. 2020 https://www.cancer.org/treatment/survivorship-during-and-after-treatment/when-cancer-doesnt-go-away.html. [Google Scholar]
- 20.McCorkle Ruth, Ercolano E, Lazenby M, Schulman-Green D, Schilling LS, Lorig K, et al. Self‐management: Enabling and empowering patients living with cancer as a chronic illness. CA. Cancer J. Clin. 2011;61(1):50–62. doi: 10.3322/caac.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikipedia. List of Hobbies. https://en.wikipedia.org/wiki/List_of_hobbies. [Google Scholar]