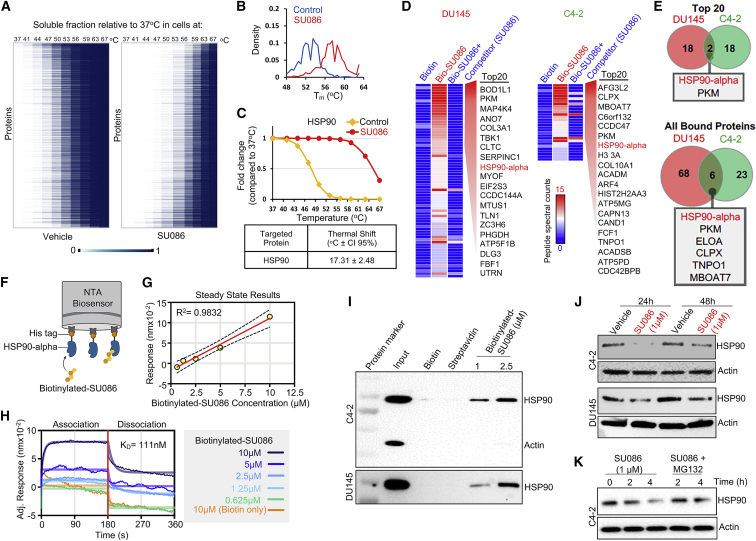
Figure 5.
Identification of HSP90 as a target of SU086 in prostate cancer cells
(A) Cellular thermal shift assay for evaluating SU086 targets in cells. DU145 cells were collected following 1.5-h incubation with DU145 cells. 1 × 106 cells were aliquoted and incubated at 10 increasing temperatures (37°C, 41°C, 44°C, 47°C, 50°C, 53°C, 56°C, 59°C, 63°C, 67°C), snap frozen, and lysed by freeze thaw cycling. Soluble proteins were tandem mass tag labeled and ran in liquid chromatography-tandem mass spectrometry analysis. Heatmap representation of thermal stability of soluble proteins in vehicle (left) or SU086 (right)-treated cells. Soluble fraction of proteins (unbound proteins) compared to the 37°C proteins was mapped as percentage (0–1) for both vehicle and SU086-treated cells.
(B) Density distribution of protein melting temperature Tm values calculated in SU086-treated and vehicle cells.
(C) Melting curve for HSP90 indicating 17.31°C shift in melting temperature in presence of SU086.
(D) Streptavidin (SA) affinity purification of biotinylated-SU086 with pretreatment with vehicle or SU086 used as a competitor followed by LC-MS/MS proteomics in C4-2 or DU145 cells. The heatmap of peptide spectral counts from pull-down samples (biotin control, biotinylated SU086, and SU086 used as a competitor). As a cutoff, we used peptide spectral counts ≤5 in the biotin only control and difference in peptide spectral counts (biotinylated SU086 group minus the SU086 with competitor group) ≥6. The top 20 proteins specifically bound to biotinylated-SU086 are listed.
(E) Venn diagram illustrates the two overlapping proteins of the top 20 pulled down proteins with biotinylated-SU086 in DU145 and C4-2 prostate cancer cells (top). Six overlapping candidate proteins that were pulled down together with SU086 consistently in DU145 and C4-2 cancer cells.
(F) Schematic representation of bio-layer interferometry (BLI) using recombinant HSP90 and biotinylated SU086. The image was created with BioRender.com.
(G) Steady-state results show the positive correlation of response (nm of wavelength shift) and concentration of biotinylated-SU086.
(H) The BLI sensorgrams were obtained using His tag-HSP90-loaded Octet NTA biosensors and serially diluted biotinylated-SU086 or biotin used as a control. The red line indicates the phases of the association (left) and dissociation (right). Equilibrium dissociation constant (KD) was calculated and obtained using GraphPad Prism equation (non-linear regression: association then dissociation) through analysis of the measurement of both association and dissociation rates sequentially. The raw signals of wavelength shift and non-linear regression curves are shown as overlays.
(I) Immunoprecipitation with biotinylated SU086 followed by western blot for HSP90 and actin as a control following treatment (1.5 h) of C4-2 or DU145 live cells with SU086 at the indicated doses.
(J) C4-2 or DU145 cells were treated with 1 μM SU086 for 24 or 48 h. Western blot analysis was performed for HSP90 and actin loading control.
(K) C4-2 cells were treated with vehicle, SU086 (1 μM), or MG132 (10 μM) + SU086 (1 μM). Cells were harvested after the indicated time points and subjected to western blot for HSP90 or actin.