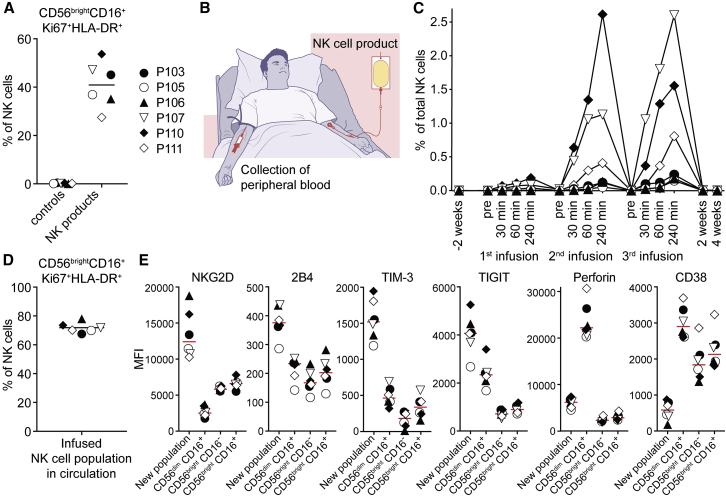
Figure 1.
Flow-cytometry-based tracking of infused autologous NK cell product in patients
(A) Percentage of ex vivo activated and expanded NK cells with the CD56brightCD16+Ki67+HLA-DR+ phenotype in the NK cell products. Controls represent study subject peripheral blood NK cells before the first infusion. Lines represent the mean values. Symbols represent patients in all panels displayed (n = 6).
(B) Strategy employed to detect ex vivo activated and expanded autologous NK cells among peripheral blood NK cells directly following the infusion.
(C) Relative size of a defined subset of the infused NK cell population as detected in the circulation of study subjects after infusion of the NK cell product. Infused NK cells were identified by their distinct phenotype by using t-SNE analysis.
(D) Percentage of NK cells with the CD56brightCD16+Ki67+HLA-DR+ phenotype within the infused cell populations followed in (C). Data shown are pooled from all time points. Line represents the mean value.
(E) Median fluorescence intensities of selected markers on the infused NK cell populations followed in (C) compared with other NK cell subpopulations. Data shown are pooled over all time points. Lines represent the median values.