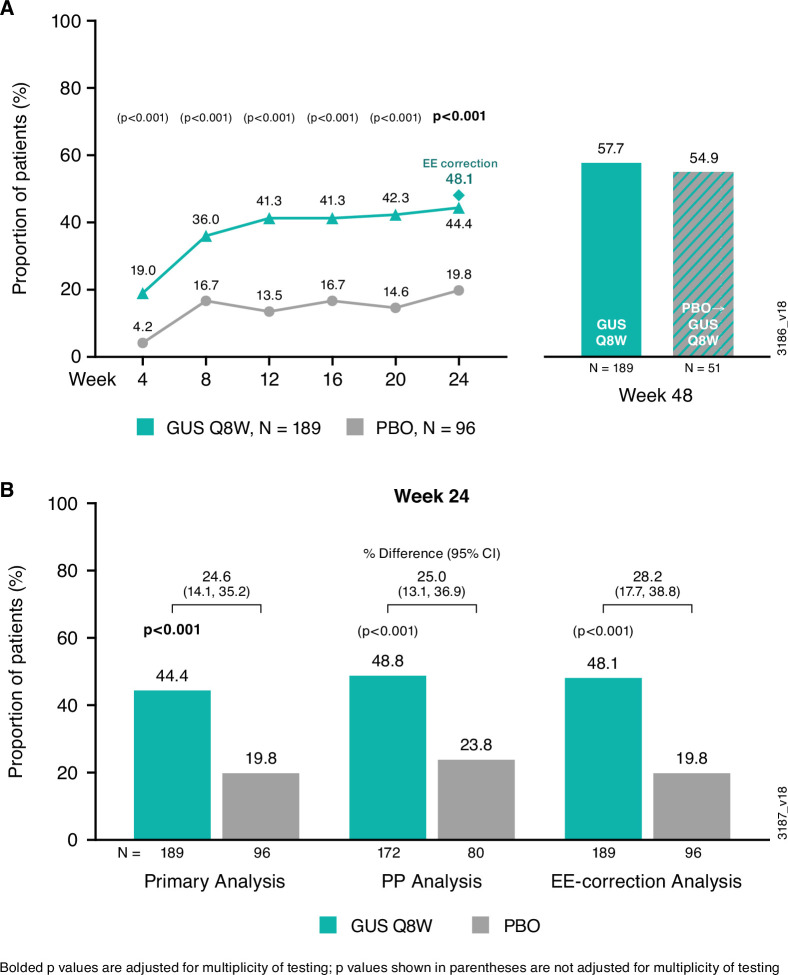
Figure 2.
ACR20 response through week 48 of COSMOS. Proportions of randomised and treated patients achieving ACR20 response through week 24 in the Primary analysis (treatment failure rules applied) (A) and ACR20 response at week 24 across the Primary, PP and EE-correction analyses (B). After week 24, analyses were performed using non-responder imputation methods, including imputation of EE patients as non-responders (see Patients and methods). Results for the placebo→guselkumab group at week 48 are reported for patients who did not enter EE and crossed over to guselkumab at week 24. ACR20, ≥20% improvement in American College of Rheumatology response criteria; EE, early escape; GUS, guselkumab; PBO, placebo; PP, per protocol; Q8W, every 8 weeks.