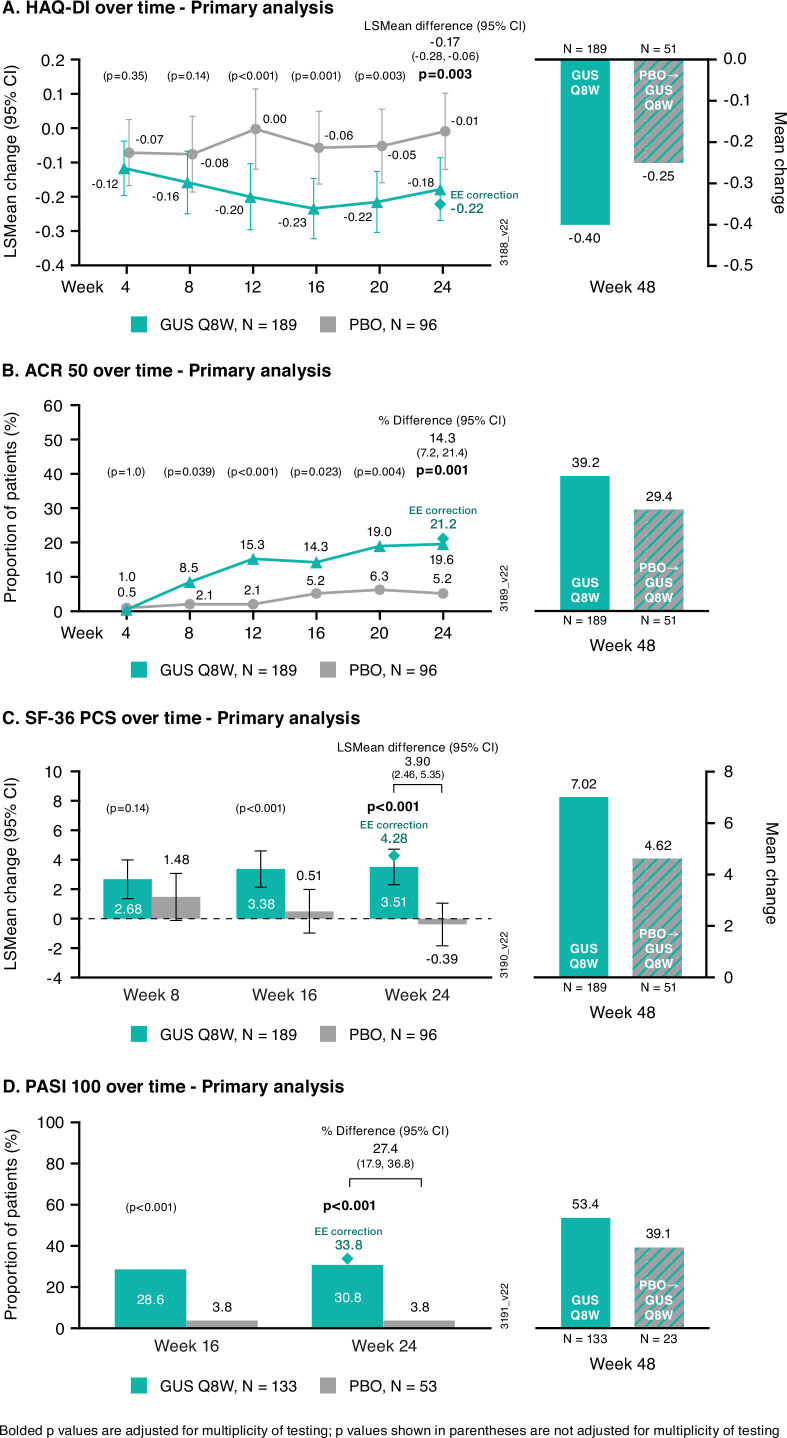
Figure 4.
Key secondary outcomes through week 48 of COSMOS. Primary analysis through week 24 and post hoc NRI analysis at week 48 of LSmean change and mean change in HAQ-DI score (A), ACR50 response (B), LSmean change and mean change in SF-36 PCS score (C), and PASI100 response (D). After week 24, analyses were performed using NRI (including imputation of EE patients as non-responders in the guselkumab group; see Patients and methods). Results for the placebo→guselkumab group at week 48 are reported for patients who did not enter EE and crossed over to guselkumab at week 24. ACR50, ≥50% improvement in American College of Rheumatology response criteria; GUS, guselkumab; HAQ-DI, Health Assessment Questionnaire-Disability Index; LS, least squares; NRI, non-responder imputation; PASI100, 100% improvement in Psoriasis Area and Severity Index; PBO, placebo; Q8W, every 8 weeks; SF-36 PCS, 36-item Short-Form Health Survey Physical Component Summary.