ABSTRACT
Background
To improve outcomes, simple screening tests are required to detect patients at increased risk of mortality. As patients with muscle weakness and wasting are at increased risk of death, we wished to review the use of the Clinical Frailty Score (CFS).
Patients and methods
Dialysis staff graded haemodialysis (HD) patients attending for routine outpatient sessions using the CFS, a functional scoring scale, for patients who require help with their instrumental activities of daily living, classified as clinically frail with scores >4, which were compared with contemporaneous Stoke–Davies comorbidity scores, post-HD body composition measured by bioimpedance, hand grip strength (HGS) and standard laboratory investigations.
Results
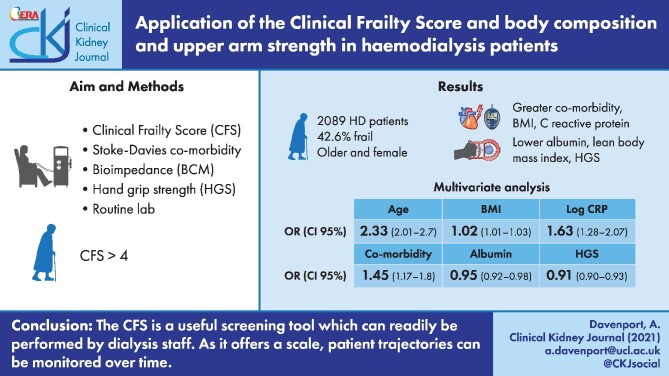
The results from 2089 patients (60.2% male) were reviewed, with 890 (42.6%) classified as frail. Frail patients were older [mean ± standard deviation (SD) 71.5 ± 15.6 versus 59.1 ± 15.6 years) and female (50.7% versus 37.3%) and had greater comorbidity {median 2 [interquartile range (IQR) 1–3] versus 1 [0–2]}, body mass index (BMI) (26.0 ± 6.7 versus 25.5 ± 5.4 kg/m2), C-reactive protein (CRP) [8 (IQR 3–20) versus 5 (2–11) mg/L], lower serum albumin (37.6 ± 4.7 versus 40.1 ± 4.7 g/L), lean BMI (8.9 ± 1.7 versus 9.7 ± 1.6 kg/m2) and HGS [13.4 (IQR 9.6–18.8) versus 20.9 (14.5–29) kg] (all P < 0.001). Frailty was independently associated in a multivariable logistic model with age {odds ratio [OR] 2.33 [95% confidence limit (CL) 2.01–2.7]}, body fat mass [OR 1.02 (CL 1.01–1.03)], log CRP [OR 1.63 (CL 1.28–2.07)] (all P < 0.001) and comorbidity [OR 1.45 (CL 1.17–1.8); P = 0.001] and negatively associated with albumin [OR 0.95 (CL 0.92–0.98) and HGS [OR 0.91 (CL 0.9–0.93)] (both P < 0.001).
Conclusion
Frail patients are at increased risk of mortality and, as such, simple reliable screening tools are required to rapidly detect patients at risk. The CFS is a useful screening tool that can be readily performed by dialysis staff to identify frail patients. Frailty in HD patients was associated with increasing age, comorbidity, fat weight and inflammation and reduced muscle strength and muscle mass. There is an overlap between frailty and both sarcopenia and protein energy wasting, which requires additional assessments, potentially including body composition, strength, dietary assessments and laboratory investigations. In addition, as the CFS offers a scale, patient trajectories can potentially be serially monitored over time, thus allowing patient-specific interventions or holistic care plans.
Keywords: bioimpedance, body mass index, co-morbidity, frailty, haemodialysis, hand grip strength, muscle mass
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The demographics of the haemodialysis (HD) population in Western Europe have changed over the past 50 years, not only in terms of increasing patient ages, but also in terms of greater comorbidity [1]. Thus, despite the many technological advances in HD [2], and improvements in general medical care and disease management, mortality remains higher than for the general population [1].
There is a natural loss of muscle mass with age, but unnatural loss of muscle mass, often termed sarcopenia, is associated with increased risk of mortality both in geriatric and HD populations [3, 4]. The European Working Group for Sarcopenia in Older People and Foundation for the National Institute of Health have developed criteria for the assessment of sarcopenia based on non-invasive measurements of muscle mass using anthropometry, bioimpedance, dual-energy X-ray absorptiometry and functional assessments of muscle strength or performance [5]. However, the prevalence of sarcopenia in both HD and peritoneal dialysis populations varies not only according to which definition of sarcopenia has been used to assess patients [6, 7], but also according to gender and ethnicity [8, 9], and whether muscle mass has been determined by anthropometry or measured by bioimpedance or other imaging [9].
HD patients differ from the general population in that the preferred vascular access, an arteriovenous fistula, alters the composition of the arm [10], and as muscle contains a high percentage of water, estimates of muscle mass change with HD [11], with a reduction in both skeletal and cardiac muscle mass reported post-HD with magnetic resonance imaging [12, 13].
Frailty has been defined as a biologic syndrome of decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, which causes vulnerability to adverse outcomes [14]. In clinical practice, frailty can be assessed using the 9-point Clinical Frailty Scale (CFS) [15] and frail HD patients have been reported to be at increased risk of both hospitalization and mortality [16]. The UK National Health Service (NHS) introduced the CFS into standard clinical practice as part of an holistic approach to patient care. As such, we wished to determine the phenotype of frailty in a multi-ethnic HD population.
MATERIALS AND METHODS
The CFS was introduced into clinical practice as part of the NHS holistic approach to patient care. The CFS is a functional assessment scale, graded 1–9. Patients with a score >4, requiring help with their instrumental activities of daily living, are classified as clinically frail [15]. The CFS assessments were made by the dialysis centre nursing staff, whereas other measurements were made by dieticians and other healthcare staff. Thus, at the time of the CFS assessments, the nursing staff were unaware of other measurements and assessments.
The electronic medical records of HD outpatients who had a CFS recorded by the dialysis centre nursing staff were reviewed, along with the corresponding post-mid-week dialysis session body composition, measured by multifrequency segmental bioimpedance (S10 and S720, InBody, Seoul, South Korea) using a standardized protocol [17]. Briefly, measurements were made after the mid-week dialysis session, after voiding if required, to minimize the potential confounding effect of pre-dialysis volume overload [18], and allowing for re-equilibration between body compartments post-dialysis [19].
Patients with bilateral amputations were excluded from the study. Hand grip strength (HGS) was measured using the hand grip-D strength dynamometer (Takei Scientific Instruments, Nigata, Japan), in accordance with the manufacturer's instructions [20]. Patients were first taught how to use the strength gauge and then measurements were made with the dynamometer held at a right angle with the elbow by the side of the body and the handle appropriately adjusted to ensure that the fingers were properly rested on the handle to perform the maximal voluntary exertion. Three measurements were made and the highest value was recorded from the non-fistula arm [21]. Routine laboratory test results were obtained from the mid-week dialysis session and the normalized protein nitrogen appearance (nPNA) rate adjusted for weight was calculated by standard methods [22]. In keeping with UK practice, the Stoke–Davies comorbidity score was used to adjudicate comorbidity [23]. This comorbidity score was developed in the UK for dialysis patients and has been reported to have a quantitative effect on their survival, independent of age and residual renal function. Ideal body weight was calculated and used to estimate the Geriatric Nutritional Risk Index (GNRI) [24]. Patient psychological distress was assessed by the distress thermometer [25, 26], a visual linear scale that was originally developed as a screening tool for UK patients with cancer and now advocated by both the UK National Institute for Clinical Excellence (NICE) and Cancer Research UK [27].
Standard dialysis treatment was post-dilution online haemodiafiltration using polysulfone dialyzers (FX series, Fresenius Medical Care, Bad Homburg, Germany) [28], Dialog+ (BBraun, Melsungen, Germany) or 4008/5008H (Fresenius Medical Care) dialysis machines, with ultrapure dialysis water quality and anticoagulation with low molecular weight heparin [29].
Statistical analysis
Results are expressed as mean ± standard deviation (SD), median and interquartile range (IQR) or percentage. Standard statistical analyses were used, including D'Agostino and Pearson normality test, Student's t-test, Mann–Whitney U-test and chi-squared test, with appropriate post hoc adjustment for multiple testing. Determinants of frailty were assessed using Spearman univariate analysis. A backward step-wise multivariable logistic regression model was then built from variables associated with frailty at P < 0.1 by Spearman univariate correlation and variables that were not statistically significant were excluded unless they improved model fit. Variables in the model were checked by collinearity and variable inflation factor. Statistical analysis was performed using Prism version 9.0 (GraphPad Software, San Diego, CA, USA) and Statistical Package for Social Science version 26.0 (IBM, Armonk, NY, USA). Statistical significance was set at ≤5%.
Ethics
This retrospective audit was checked and complied with the UK NHS Health Research Authority guidelines for clinical audit and service development and with UK NICE best practices with all patient data anonymized prior to analysis (https://www.hra.nhs.uk).
RESULTS
The CFS was recorded by the dialysis centre nursing staff in 2089 HD patients of a potential 2224 (94%) (Table 1) dialysing in four dialysis centres under the care of a UK university hospital. Exclusions included recent non-elective hospital admissions, patients who recovered residual renal function and those starting dialysis within 90 days. In total, 42.6% of patients were classified as frail with a CFS score >4. Frail patients were older and more often female. More South Asian patients were classified as frail compared with patients from other ethnicities. Frail patients had greater overall comorbidity scores and more frail patients had a history of diabetes and cardiovascular disease. However, fewer frail patients were prescribed antihypertensive medications and were less likely to be current smokers. Self-reported psychological distress was recorded in 1740 (83.3%) patients, using the distress thermometer, with exclusions due to dementia and language barriers. Self-reported psychological distress cases were not greater in the frail group.
Table 1.
Patient demographics, dialysis vintage (dialysis months), dialysis adequacy (urea reduction ratio), nPNA, Stoke–Davies comorbidity score (Davies comorbidity), myocardial infarction (MI), coronary artery bypass graft surgery (CABG), coronary artery stenting (stent), peripheral vascular disease (PVD), aortic or iliac artery aneurysm or carotid artery stenosis (CVD), transient ischaemic attack (TIA), antihypertensive medications (BP meds) and psychological distress (distress thermometer)
| Variable | All patients | Not frail | Frail |
|---|---|---|---|
| Patients, n | 2089 | 1199 | 890 |
| Male, n (%) | 1256 (60.2) | 788 (65.7) | 468 (52.6)*** |
| Age (years), mean ± SD | 64.6 ± 16.6 | 59.1 ± 15.6 | 71.5 ± 12.3*** |
| White, n (%) | 898 (43.3) | 525 (25.3) | 373 (18) |
| Black, n (%) | 567 (27.4) | 337 (16.3) | 230 (11.5) |
| South Asian, n (%) | 453 (21.9) | 237 (11.4) | 216 (10.4)*** |
| East Asian, n (%) | 136 (6.6) | 93 (4.5) | 43 (2.1) |
| Weight (kg), mean ± SD | 70.6 ± 18.5 | 71.1 ±17.0 | 70.0 ± 20.1 |
| BMI (kg/m2), mean ± SD | 25.7 ± 6.0 | 25.5 ±5.4 | 26.0 ± 6.7*** |
| Dialysis (months), median (IQR) | 29.8 (12.2–65.3) | 26.1 (10.9–56.1) | 36.4 (13.7–72.8)*** |
| Urea reduction ratio (%), mean ± SD | 75.7 ± 9.5 | 76.0 ±9.4 | 75.4 ± 9.5 |
| nPNA (g/kg/day), median (IQR) | 1.14 (0.96–1.4) | 1.18 (1.01–1.44) | 1.1 (0.92–1.34)*** |
| Davies comorbidity, median (IQR) | 1 (1–2) | 1 (0–2) | 2 (1–3)*** |
| Diabetes mellitus, % | 44.3 | 34.3 | 58.6 *** |
| MI/CABG/stent, % | 15.6/8/9.6 | 13.7/5.6/7.9 | 20***/11***/12** |
| PVD/CVD, % | 9.8/3.7 | 7/2.8 | 13.5***/5** |
| TIA/stroke, % | 2.4/12.3 | 2.3/7.9 | 2.7/18.3*** |
| Prescribed BP meds, n (%) | 1295 (62.4) | 796 (66.6) | 499 (56.6)*** |
| BP meds (n), median (IQR) | 1 (0–1.25) | 1 (0–2) | 1(0–1)*** |
| Cancer/active cancer, % | 15.3/5.2 | 14.8/4.4 | 15.9/6.3 |
| Smoker/ex-smoker, % | 15.9/32 | 20/31.1 | 10.1***/33.2 |
| Distress thermometer, median (IQR) | 4 (1–5) | 3 (1–5) | 4 (1–6) |
**P < 0.01 and ***P < 0.001 non-frail versus frail.
Frail patients had been treated by HD longer, but dialysis adequacy as determined by the urea reduction ratio was similar. Although body weight was similar, frail patients had greater body mass index (BMI), with corresponding greater fat weight and lower lean body mass (Figure 1). HGS was recorded in 1783 (85.4%) patients. HGS could not be recorded in patients with finger amputations, severe carpal tunnel syndrome and upper limb paralysis and in 15 patients who declined dietitian review. Frail patients had lower grip strength (Figure 1).
Figure 1:
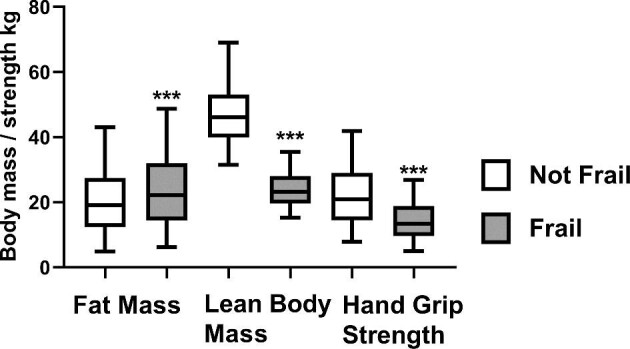
Comparison of fat mass and lean body mass measured by multifrequency bioimpedance post-mid-week HD session and HGS in frail patients (CFS >4) and non-frail patients. ***P < 0.0001 versus non-frail.
Laboratory tests revealed that frail patients had lower haemoglobin, albumin, urea, creatinine, phosphate and cholesterol concentrations and increased C-reactive protein (CRP), N-terminal brain natriuretic peptide (NT-proBNP), bicarbonate, glucose and corrected calcium (Table 2). Both estimates of dietary protein intake (nPNA) and GNRI scores were lower in frail patients.
Table 2.
Standard laboratory investigations
| Investigation | All patients | Not frail | Frail |
|---|---|---|---|
| Haemoglobin (g/L) | 109 ± 14 | 110 ± 14 | 108 ± 14** |
| Albumin (g/L) | 39.9 ± 4.9 | 40.1 ± 4.7 | 37.6 ± 4.7*** |
| Bicarbonate (mmol/L) | 21.5 ± 2.7 | 21.3 ± 2.7 | 21.8 ± 2.6*** |
| Sodium (mmol/L) | 139 ± 5 | 139 ± 3 | 138 ± 6** |
| Potassium (mmol/L) | 5.1 ± 0.7 | 5.8 ± 0.7 | 4.9 ± 0.7*** |
| Calcium (mmol/L) | 2.31 ± 0.17 | 2.30 ± 0.17 | 2.34 ± 0.16*** |
| Phosphate (mmol/L), median (IQR) | 1.63 (1.32–1.99) | 1.72 (1.41–2.1) | 1.52 (1.23–1.84)*** |
| CRP (mg/L), median (IQR) | 4 (1–13) | 5 (2–11) | 8 (3–20)*** |
| NT-proBNP (ng/L), median (IQR) | 3830 (1524–12 130) | 3161 (1032–9223 | 3985 (1426–13 079)** |
| Cholesterol (mmol/L), median (IQR) | 3.9 (3.1–4.4) | 3.8 (3.1–4.5) | 3.5 (2.0–4.2)*** |
| Glucose (mmol/L), median (IQR) | 6.1 (5.1–7.5) | 6.3 (5.3–7.9) | 7.1 (5.7–9.2)*** |
| Urea (mmol/L) | 20.0 ± 6.5 | 20.6 ± 6.3 | 18.8 ± 6.4*** |
| Creatinine (µmol/L), median (IQR) | 740 (572–978) | 794 (631–990) | 611 (490–735)*** |
| GNRI | 98.4 ± 8.0 | 100.2 ± 7.5 | 96.1 ± 8.0*** |
| ECW/TBW | 0.396 ± 0.018 | 0.390 ± 0.019 | 0.405 ±0.017*** |
Values expressed as mean ± SD unless stated otherwise.
**P < 0.01 and ***P < 0.001 female versus male.
Post-dialysis bioimpedance measurement of the extracellular water (ECW): total body water (TBW) ratio was higher in frail patients, but frail patients had lower intracellular water (ICW) (20.1 ± 5.0 versus 22.5 ± 5.3 L; P < 0.001).
On univariate analysis, CFS was positively associated with ECW:TBW ratio, age, comorbidity, CRP, body fat mass (BFM) and NTproBNP and negatively associated with HGS, serum albumin, nPNA, haemoglobin, serum electrolytes and cholesterol, TBW and weight (Table 3).
Table 3.
Variables statistically associated with Rockwood CFS on univariate analysis
| Variable | Spearman's rho | P-value |
|---|---|---|
| ECW:TBW ratio | 0.51 | <0.001 |
| Age (years) | 0.479 | <0.001 |
| Stoke–Davies comorbidity score | 0.353 | <0.001 |
| CRP (mg/L) | 0.233 | <0.001 |
| BFM (kg) | 0.161 | <0.001 |
| Serum adjusted calcium (mmol/L) | 0.127 | <0.001 |
| NT-proBNP (ng/L) | 0.105 | <0.001 |
| HGS (kg) | –0.474 | <0.001 |
| Serum albumin (g/L) | –0.366 | <0.001 |
| Fat free mass (kg) | –0.281 | <0.001 |
| TBW (L) | –0.270 | <0.001 |
| Normalized protein nitrogen accumulation rate (g/kg/day) | –0.236 | <0.001 |
| Serum phosphate (mmol/L) | –0.205 | <0.001 |
| Serum cholesterol (mmol/L) | –0.185 | <0.001 |
| Serum sodium (mmol/L) | –0.165 | <0.001 |
| Serum potassium (mmol/L) | –0.137 | <0.001 |
| Haemoglobin (g/L) | –0.107 | <0.001 |
| Post-dialysis weight | –0.093 | <0.001 |
A multivariable logistic model was then created and frailty was independently positively associated with older age, increasing comorbidity, BFM and CRP and negatively associated with HGS and serum albumin (Table 4).
Table 4.
Multivariable logistic regression model of variables independently associated with the Rockwood CFS
| Variable | β | S.E. β | Wald | OR | 95% confidence limit | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 0.846 | 0.075 | 125.8 | 2.33 | 2.01–2.7 | <0.001 |
| Comorbidity | 0.37 | 0.11 | 11.2 | 1.45 | 1.17–1.80 | 0.001 |
| BFM (kg) | 0.019 | 0.005 | 12.6 | 1.02 | 1.01–1.03 | <0.001 |
| Albumin (g/L) | –0.052 | 0.014 | 13.1 | 0.95 | 0.92–0.98 | <0.001 |
| Log CRP | 0.488 | 0.122 | 16.1 | 1.63 | 1.28–2.07 | <0.001 |
| HGS (kg) | –0.09 | 0.009 | 110.3 | 0.91 | 0.90–0.93 | <0.001 |
Comorbidity: Stoke–Davies comorbidity score; S.E. β: standard error β; Adjusted r2 = 0.382.
DISCUSSION
HD patients are at greater risk of mortality. As such, detecting patients at increased risk to potentially allow for earlier intervention is to be welcomed. Although sarcopenia is recognized to be associated with mortality [3], there is no agreed upon definition and diagnosis requires a step-wise approach based on the measurement of muscle mass and an assessment of muscle function [5]. Dialysis patients may be at an increased risk of a loss of both muscle and fat mass (cachexia), and as chronic kidney disease is an inflammatory condition, others have introduced the concept of protein energy wasting (PEW) [4]. Again, the definition of PEW is based on the combination of results of laboratory tests, changes in body composition and formal assessments of dietary intake. The 9-point CFS allows for rapid clinical scoring of patients based on functional ability that can readily be performed in the outpatient setting or by dialysis nursing staff without requiring the measurement of muscle strength, body composition, nutritional intake or laboratory investigations [30].
The prevalence of frailty in our patient cohort was slightly greater than 40%. As probably expected, frail patients were older, with greater comorbidity, particularly diabetes and cardiovascular diseases. More female patients were frail, in keeping with reports of a greater prevalence for sarcopenia in female dialysis patients [8]. In addition, we noted relatively more frailty in patients of South Asian ethnicity compared with those of White and Black ethnicities. The proportion of East Asian patients was too low in this study to be definitive, but frailty did not appear to differ between the White and Black cohorts.
Although frail and non-frail patients received the same sessional dialyser urea clearance, frail patients had a lower dietary protein intake, as estimated by nPNA, and a lower GNRI. The higher serum bicarbonate in frail group could reflect lower dietary protein intake with reduced production of acids. This reduction in dietary intake demonstrates an overlap between frailty and PEW [4].
The lower serum phosphate, potassium and cholesterol noted in frail patients would be in keeping with reduced nutritional intake. Although adjusted serum calcium was higher in our frail patients, this may be artefactual, after adjusting the serum calcium for the lower serum albumin. Observational studies in dialysis patients have reported increased mortality in those with lower predialysis sodium and potassium [31, 32]. This could reflect a lower nutritional state or could be secondary to dilution and inflammation. Other studies have shown an association with inflammation and increased ECW:TBW ratios [33]. Although an increased ECW:TBW ratio can reflect ECW expansion, this ratio can also be increased by a loss of ICW, and ICW was lower in the frail group. Whereas post-HD weight was similar, frail patients had lower muscle mass but increased body fat. For the whole cohort there was a negative association between lean mass and appendicular mass indexed for height and percentage body fat (r = 0.2, P < 0.001). Previous observational studies have noted an increased prevalence of sarcopenic obesity in HD patients, particularly for female patients, due to the combination of reduced muscle mass and increased fat mass [34]. This study supports these earlier reports, as more female patients were classed as being frail. As such, this highlights the potential confounding of using BMI as an assessment of nutritional status in dialysis patients, due to changes in muscle and fat mass in dialysis patients. Observational studies have reported a survival advantage for obese dialysis patients. Classically, dialysis adequacy is calculated using an anthropometric estimate of body water (V) based on patient weight. As fat is less metabolically active and contains less water than muscle, this can potentially lead to an overestimation of V and thus obese patients receiving a greater amount of dialysis for their actual amount of TBW [35, 36]. Although our frail patients had a greater BMI, the mean BMI was only 26, and as such, frail patients were not obese. In keeping with sarcopenia, our frail patients not only had less muscle mass, but also had reduced muscle function as assessed by HGS.
Frail patients had higher CRP values and lower serum albumin, suggesting a greater inflammatory response, thus overlapping with PEW. Greater inflammation would reduce the response to erythropoietin-stimulating agents, thus accounting for the lower haemoglobin. Similarly, although the ECW:TBW ratio and NT-proBNP are associated with heart failure and volume overload, inflammation increases vascular permeability, thus increasing extravascular fluid retention and both the ECW:TBW ratio and NT-proBNP [37].
In this cross-sectional study, frailty scores were taken at a single time point along with corresponding assessments of body composition, upper arm strength and laboratory investigations. Additional studies are required to review changes in body composition and muscle strength with changes in CFS. However, as frailty in both older patients and HD patients is associated with an increased risk of both hospitalization and mortality [16, 38], detecting frailty has clinical relevance. Similarly, patients with sarcopenia and those with PEW are at increased risk of mortality [3, 4]. However, there are several definitions of sarcopenia [39–41], and to fulfil the definitions, measurements of body composition are required along with a functional assessment, such as HGS; similarly body composition, along with other criteria, including dietary assessments and laboratory tests, is required to determine whether a patient has PEW [3, 5]. In everyday clinical practice, not all centres may have the necessary equipment or personnel to undertake these assessments on a routine basis. There is considerable overlap between these phenotypes, but applying the CFS is less time-consuming, requires no additional measurements or investigations and can be readily assessed by healthcare workers [30]. In addition, irrespective of whether patients are classified as having sarcopenia or PEW, the CFS is a scale that potentially allows serial monitoring of patients over time to determine any improvement or deterioration in patient trajectories. Following the introduction of the CFS into routine clinical practice as part of a holistic approach to patient care in the UK, we characterized frailty in our HD patients to determine whether the CFS is equally applicable to HD patients as a screening tool to detect patients with frailty. CFS assessments made by the dialysis centre nursing staff demonstrated that frailty is associated with age, inflammation, increasing comorbidity, changes in body composition and loss of muscle strength and these assessments can be rapidly made in routine clinical practice [30]. Thus the study demonstrates that the introduction of the CFS into the routine care of HD patients allows the rapid detection of patients with frailty, thus potentially highlighting patients who would benefit from active intervention or a more personalized therapy plan at an earlier stage rather than waiting for the additional assessments and tests required to confirm or refute sarcopenia and PEW.
ACKNOWLEDGEMENTS
We thank D. Febriani for her statistical analyses and the medical and allied dialysis professionals at the Royal Free Hospital chronic kidney disease centres. This retrospective audit complied with UK National Research Ethics Services (NRES) guidelines; formal ethics approval by the NRES was not required.
AUTHORS’ CONTRIBUTIONS
A.D. wrote the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part or in an abstract format.
DATA AVAILABILITY STATEMENT
Data are held by the UCL Department of Nephrology V drive and are available upon reasonable request and within NHS guidelines.
REFERENCES
- 1. Storey BC, Staplin N, Harper CHet al. . Declining comorbidity-adjusted mortality rates in English patients receiving maintenance renal replacement therapy. Kidney Int 2018; 93: 1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davenport A. New dialysis technology and biocompatible materials. Contrib Nephrol 2017; 189:130–136 [DOI] [PubMed] [Google Scholar]
- 3. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019; 393: 2636–2646 [DOI] [PubMed] [Google Scholar]
- 4. Hanna RM, Ghobry L, Wassef Oet al. . A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif 2020; 49: 202–211 [DOI] [PubMed] [Google Scholar]
- 5. Slee A, McKeaveney C, Adamson Get al. . Estimating the prevalence of muscle wasting, weakness, and sarcopenia in hemodialysis patients. J Ren Nutr 2020; 30: 313–321 [DOI] [PubMed] [Google Scholar]
- 6. Tangvoraphonkchai K, Hung R, Sadeghi-Alavijeh Oet al. . Differences in prevalence of muscle weakness (sarcopenia) in haemodialysis patients determined by hand grip strength due to variation in guideline definitions of sarcopenia. Nutr Clin Pract 2018; 33: 255–260 [DOI] [PubMed] [Google Scholar]
- 7. Abro A, Delicata LA, Vongsanim Set al. . Differences in the prevalence of sarcopenia in peritoneal dialysis patients using hand grip strength and appendicular lean mass: depends upon guideline definitions. Eur J Clin Nutr 2018; 72: 993–999 [DOI] [PubMed] [Google Scholar]
- 8. Yoowannakul S, Tangvoraphonkchai K, Vongsanim Set al. . Differences in the prevalence of sarcopenia in haemodialysis patients: the effects of gender and ethnicity. J Hum Nutr Diet 2018; 31: 689–696 [DOI] [PubMed] [Google Scholar]
- 9. Tangvoraphonkchai K, Hung R, Sadeghi-Alavijeh Oet al. . Differences in prevalence of muscle weakness (sarcopenia) in haemodialysis patients determined by hand grip strength due to variation in guideline definitions of sarcopenia. Nutr Clin Pract 2018; 33: 255–260 [DOI] [PubMed] [Google Scholar]
- 10. Maharjan SRS, Jiang K, Slee Aet al. . Comparison of multifrequency bioimpedance measured lean mass to that calculated from anthropomometric measurements in patients with chronic kidney disease. Eur J Clin Nutr 2019; 73: 1200–1202 [DOI] [PubMed] [Google Scholar]
- 11. Al-Joudi E, Slee A, Davenport A. The effect of an arteriovenous fistula and haemodialysis on anthropometric measurements of the upper arm. Eur J Clin Nutr 2020; 74: 1240–1242 [DOI] [PubMed] [Google Scholar]
- 12. Tangvoraphonkchai K, Davenport A. Changes in body composition following haemodialysis as assessed by bioimpedance spectroscopy. Eur J Clin Nutr 2017; 71: 169–172 [DOI] [PubMed] [Google Scholar]
- 13. Sawant A, House AA, Chesworth BMet al. . Association between muscle hydration measures acquired using bioelectrical impedance spectroscopy and magnetic resonance imaging in healthy and hemodialysis population. Physiol Rep 2015; 3: e12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotecha T, Martinez-Naharro A, Yoowannakul Set al. . Acute changes in cardiac structural and tissue characterisation parameters following haemodialysis measured using cardiovascular magnetic resonance. Sci Rep 2019; 9: 1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fried LP, Tangen CM, Walston Jet al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156 [DOI] [PubMed] [Google Scholar]
- 16. Rockwood K, Song X, MacKnight Cet al. . A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McAdams-DeMarco MA, Law A, Salter MLet al. . Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 2013; 61: 896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fürstenberg A, Davenport A. Comparison of multifrequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry assessments in outpatient haemodialysis patients. Am J Kidney Dis 2010; 57: 123–129 [DOI] [PubMed] [Google Scholar]
- 19. El-Kateb S, Davenport A. Changes in intracellular water following hemodialysis treatment lead to changes in estimates of lean tissue using bioimpedance spectroscopy. Nutr Clin Pract 2016; 31: 375–377 [DOI] [PubMed] [Google Scholar]
- 20. Tangvoraphonkchai K, Davenport A. Changes in body composition following haemodialysis as assessed by bioimpedance spectroscopy. Eur J Clin Nutr 2017; 71: 169–172 [DOI] [PubMed] [Google Scholar]
- 21. Omichi Y, Srivareerat M, Panorchan Ket al. . Measurement of muscle strength in haemodialysis patients by pinch and hand grip strength and comparison to lean body mass measured by multifrequency bio-electrical impedance. Ann Nutr Metab 2016; 68: 268–275 [DOI] [PubMed] [Google Scholar]
- 22. Jiang K, Singh Maharjan SR, Slee Aet al. . Differences between anthropometric and bioimpedance measurements of muscle mass in the arm and hand grip and pinch strength in patients with chronic kidney disease. Clin Nutr 2021; 40: 320–332 [DOI] [PubMed] [Google Scholar]
- 23. National Kidney Foundation . KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930 [DOI] [PubMed] [Google Scholar]
- 24. Davies SJ, Phillips L, Naish PFet al. . Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002; 17: 1085–1092 [DOI] [PubMed] [Google Scholar]
- 25. Bouillanne O, Morineau G, Dupont Cet al. . Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783 [DOI] [PubMed] [Google Scholar]
- 26. Camilleri S, Chong S, Tangvoraphonkchai Ket al. . Effect of self-reported distress thermometer score on the maximal handgrip and pinch strength measurements in hemodialysis patients. Nutr Clin Pract 2017; 32: 682–686 [DOI] [PubMed] [Google Scholar]
- 27. Maharjan SRS, Davenport A. The effects of supported shared-care and hemodialysis self-care on patient psychological well-being, interdialytic weight gain, and blood pressure control. Hemodial Int 2020; 24: 29–35 [DOI] [PubMed] [Google Scholar]
- 28. National Institute for Clinical Excellence . https://www.evidence.nhs.uk/search?ps=250&q=distress+thermometer (10 October 2021, date last accessed)
- 29. Tangvoraphonkchai K, Riddell A, Davenport A. Platelet activation and clotting cascade activation by dialyzers designed for high volume online hemodiafiltration. Hemodial Int 2018; 22: 192–200 [DOI] [PubMed] [Google Scholar]
- 30. Davenport A. The rationale for the use of low molecular weight heparin for hemodialysis treatments. Hemodial Int 2013; 17(Suppl 1): S28–S32 [DOI] [PubMed] [Google Scholar]
- 31. Nixon AC, Brown J, Brotherton Aet al. . Implementation of a frailty screening programme and geriatric assessment service in a nephrology centre: a quality improvement project. J Nephrol 2020;34: 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Chidadi A, Nitsch D, Davenport A. The effect of serum sodium on survival in patients treated by peritoneal dialysis in the United Kingdom. Perit Dial Int 2017; 37: 70–77 [DOI] [PubMed] [Google Scholar]
- 33. Kovesdy CP, Regidor DL, Mehrotra Ret al. . Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2007; 2: 999–1007 [DOI] [PubMed] [Google Scholar]
- 34. Chhabra R, Davenport A. Prehemodialysis hyponatremia and extracellular water: is it simply too much water ? Ther Apher Dial 2021; doi: 10.1111/1744-9987.13685. [DOI] [PubMed] [Google Scholar]
- 35. Bellafronte NT, Ono AQM, Chiarello PG. Sarcopenic obesity in chronic kidney disease: challenges in diagnosis using different diagnostic criteria. Med Princ Pract 2021; 30: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davenport A. Differences in prescribed Kt/V and delivered haemodialysis dose—why obesity makes a difference to survival for haemodialysis patients when using a ‘one size fits all’ Kt/V target. Nephrol Dial Transplant 2013; 28(Suppl 4): iv219–iv223 [DOI] [PubMed] [Google Scholar]
- 37. Sridharan S, Vilar E, Davenport Aet al. . Scaling hemodialysis target dose to reflect body surface area, metabolic activity, and protein catabolic rate: a prospective, cross-sectional study. Am J Kidney Dis. 2017; 69 : 358–366 [DOI] [PubMed] [Google Scholar]
- 38. Booth J, Pinney J, Davenport A. N-terminal proBNP—marker of cardiac dysfunction, fluid overload, or malnutrition in hemodialysis patients? Clin J Am Soc Nephrol 2010; 5: 1036–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alfaadhel TA, Soroka SD, Kiberd BAet al. . Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 2015; 10: 832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abro A, Delicata LA, Vongsanim Set al. . Differences in the prevalence of sarcopenia in peritoneal dialysis patients using hand grip strength and appendicular lean mass: depends upon guideline definitions. Eur J Clin Nutr 2018; 72: 993–999 [DOI] [PubMed] [Google Scholar]
- 41. Yoowannakul S, Tangvoraphonkchai K, Vongsanim Set al. . Differences in the prevalence of sarcopenia in haemodialysis patients: the effects of gender and ethnicity. J Hum Nutr Diet 2018; 31: 689–696 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are held by the UCL Department of Nephrology V drive and are available upon reasonable request and within NHS guidelines.