Figure 1.
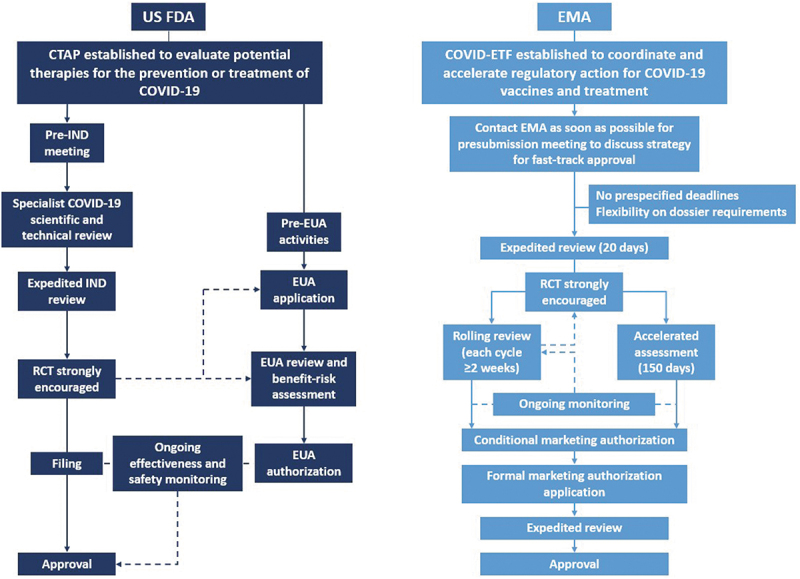
Regulatory pathways adopted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) during the COVID-19 pandemic. COVID-19, coronavirus disease 2019; COVID-ETF: COVID-19 EMA pandemic Task Force; CTAP: Coronavirus Treatment Acceleration Program; EUA: Emergency Use Authorization; IND: investigational new drug; RCT: randomized controlled trial.
