Fig. 1.
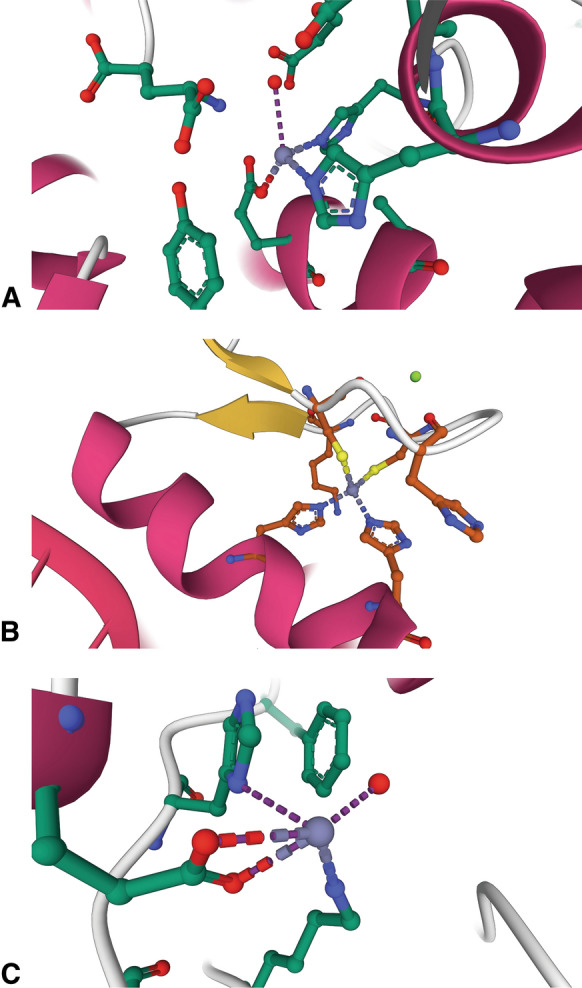
The diversity of Zn2+ binding sites in protein by type. a Catalytic. Aminopeptidase A/glutamyl aminopeptidase (4KXC) contains a catalytic Zn2+ ion bound by 3 protein ligands (2 histidines and a glutamate residue) and a water molecule that coordinate the ion in a tetrahedral geometry (Yang et al. 2013). b Structural. The Zn2+ binding site of the Cys2His2 zinc finger of TFIIIA (1UN6) (Lu et al. 2003) illustrates an example of a structural Zn2+ ion. c Regulatory. Caspase-6 (4FXO) contains a regulatory Zn2+ ion, in which the bound metal is coordinated in a distorted tetrahedral geometry through interactions with histidine, lysine, and glutamate residues. A water molecule is the fourth ligand. The glutamate is coordinated to the Zn2+ ion through a bidentate interaction (Velazquez-Delgado and Hardy 2012). Protein structure figures were created with Mol* Viewer (Sehnal et al. 2021) and RCSB PDB
