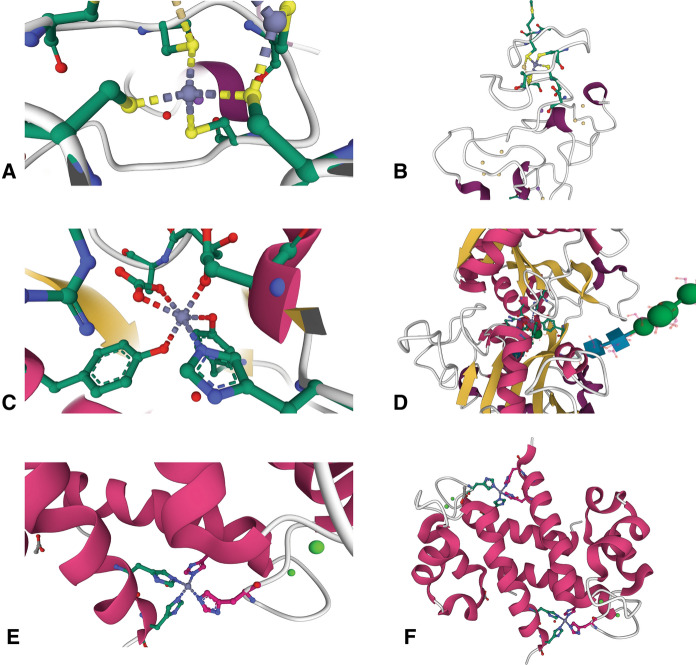
Fig. 3.
Zinc binding sites in metal carrier proteins. a One of the Zn2+ binding sites in the β-domain of rat metallothionein-2 (4MT2) shows the ion coordinated in a tetrahedral geometry by four cysteine residues (Braun et al. 1992). b This Zn2+ binding site in MT2 resides in a loop near the protein’s outer surface, shown near the top of the panel. c Structure of the Zn2+ binding site in the C-terminal lobe of lactoferrin (1SDX), showing an octahedral coordination geometry. There are 6 ligands, which include a histidine, two tyrosines, and an asparate residue. The remaining two ligands are contributed through a bidentate interaction with a carbonate ion (Jabeen et al. 2005). d The Zn2+ binding site of LTF is located near the center of the protein, accessible through a wide cleft. e The S100A8 homodimer (5HLV) contains two Zn2+ binding sites consisting of four histidine residues, two from each monomer, that coordinate the ion in a tetrahedral geometry (Lin et al. 2016). f The two Zn2+ binding sites of S100A8 are located on either side of the molecule at the dimer interface at the periphery of the complex. Protein structure figures were created with Mol* Viewer (Sehnal et al. 2021) and RCSB