Abstract
Objective
Numerous investigators have theorized that postoperative changes in Alzheimer's disease neuropathology may underlie postoperative neurocognitive disorders. Thus, we determined the relationship between postoperative changes in cognition and cerebrospinal (CSF) tau, p‐tau‐181p, or Aβ levels after non‐cardiac, non‐neurologic surgery in older adults.
Methods
Participants underwent cognitive testing before and 6 weeks after surgery, and lumbar punctures before, 24 h after, and 6 weeks after surgery. Cognitive scores were combined via factor analysis into an overall cognitive index. In total, 110 patients returned for 6‐week postoperative testing and were included in the analysis.
Results
There was no significant change from before to 24 h or 6 weeks following surgery in CSF tau (median [median absolute deviation] change before to 24 h: 0.00 [4.36] pg/mL, p = 0.853; change before to 6 weeks: −1.21 [3.98] pg/mL, p = 0.827). There were also no significant changes in CSF p‐tau‐181p or Aβ over this period. There was no change in cognitive index (mean [95% CI] 0.040 [−0.018, 0.098], p = 0.175) from before to 6 weeks after surgery, although there were postoperative declines in verbal memory (−0.346 [−0.523, −0.170], p = 0.003) and improvements in executive function (0.394, [0.310, 0.479], p < 0.001). There were no significant correlations between preoperative to 6‐week postoperative changes in cognition and CSF tau, p‐tau‐181p, or Aβ42 changes over this interval (p > 0.05 for each).
Interpretation
Neurocognitive changes after non‐cardiac, non‐neurologic surgery in the majority of cognitively healthy, community‐dwelling older adults are unlikely to be related to postoperative changes in AD neuropathology (as assessed by CSF Aβ, tau or p‐tau‐181p levels or the p‐tau‐181p/Aβ or tau/Aβ ratios).
Trial Registration: clinicaltrials.gov (NCT01993836).
Introduction
One of the largest controversies in perioperative medicine over the last quarter century has been whether anesthesia and surgery contribute to long‐term cognitive decline and/or the development of dementia in older adults. This question has major public health implications, since approximately half of adults over 65 will undergo at least one surgery, and over 120,000 Americans will die of Alzheimer's disease (AD) per year. 1 , 2 Thus, if anesthesia and surgery make even a small contribution to long‐term cognitive decline or increased AD risk, this would have significant population‐level implications.
Four lines of evidence raise the possibility that anesthesia and surgery may accelerate cognitive decline and/or the underlying neuropathologic processes associated with AD. First, the two largest epidemiologic studies on this topic have found increased dementia rates in people who underwent surgery versus those who did not, though these studies were retrospective 3 , 4 and smaller studies have not replicated these associations. 5 , 6 , 7 , 8 , 9
Second, cognitive decline occurs in many older adults within months after anesthesia and surgery. 10 This early cognitive decline, typically termed postoperative cognitive dysfunction or neurocognitive disorder postoperative 11 when accompanied by subjective cognitive complaints, has been associated with long‐term cognitive decline 5 years later. 12 Similarly, postoperative delirium has been associated with greater cognitive decline 3 years later. 13 However, in both cases, it remains unclear to what extent this long‐term cognitive decline reflects an effect of anesthesia and surgery (or other aspects of perioperative care such as sleep disruption, pain, or inflammation) versus confounding due to underlying surgical disease processes or medical comorbidities (e.g., cardiovascular disease). 14 , 15 Nonetheless, cognitive decline within 1–3 months after surgery has been associated with functional brain connectivity changes similar to those seen in early mild cognitive impairment and dementia due to AD. 16 , 17 , 18 Postoperative cortical thinning has also been observed within brain regions that show atrophy in AD, 19 and patients with MCI show greater hippocampal volume loss after surgery than those who are cognitively normal. 20
Third, in mouse models and in vitro systems, anesthetic drugs and surgical stress promote many key pathophysiologic processes involved in AD such as amyloid beta (Aβ) plaque formation 21 , 22 and tau phosphorylation. 23 , 24 , 25 , 26 Blocking these processes in mouse models improves memory following surgery. 27 , 28 Fourth, we and others have found postoperative increases in CSF tau levels into the range seen in AD. 29 , 30 , 31 , 32 , 33 However, CSF tau level increases are seen in multiple neurologic disorders aside from AD; tau is best considered a pan‐neuronal injury marker. 34 , 35 Based on these four lines of evidence, we undertook this prospective cohort study to determine the relationship between postoperative changes in both cognition and CSF Aβ, tau, and p‐tau‐181p levels in older surgical patients.
Methods
Study participants
Markers of Alzheimer's Disease and neuroCognitive Outcomes after Perioperative Care (MADCO‐PC) was an IRB‐approved observational cohort study with a nested randomized controlled trial, in which all cohort study patients were also randomized to isoflurane versus propofol for anesthetic maintenance. We designed the study this way since virtually all patients currently undergoing general anesthesia receive either an inhaled anesthetic (with isoflurane being the most common at our institution) or propofol, both of which are considered standard of care. Thus, randomizing the parent cohort study patients to isoflurane versus propofol does not expose these patients to any drug outside the current standard of care. Further, randomizing patients to isoflurane versus propofol reduces other factors that could bias which drug a patient receives, thus allowing for an unbiased comparison of the effects of these two drugs on cognition and CSF AD‐related biomarkers in older surgical patients.
The prespecified primary study outcome was on the relationship between cognitive and CSF tau change over the interval from before to 6 weeks after surgery in the full observational cohort. The effect of intraoperative anesthetic randomization (i.e., in the nested RCT) is a secondary outcome described in a separate manuscript now under preparation. MADCO‐PC was conducted at Duke University Medical Center (Durham, NC), and registered with clinicaltrials.gov (NCT01993836). Eligibility criteria were age ≥60 years, undergoing major non‐cardiac, non‐neurologic surgery (i.e., under general anesthesia for a planned intraoperative duration of >2 h and an expected hospital stay of ≥1 night), English‐speaking, and ability to give informed consent. Patients were excluded if they were an inmate of a correctional facility, pregnant, had a family or personal history of malignant hyperthermia, were unable to receive propofol/isoflurane due to allergy or other specific contraindication, or were taking anticoagulants that would preclude safe lumbar puncture. 36 , 37 Patients who received systemic chemotherapy between preoperative and 6‐week postoperative cognitive testing were also excluded to avoid confounding effects on cognition. There were no exclusions for preoperative/baseline cognitive status.
The Duke Hospital electronic surgery schedule was screened for patients potentially eligible for the study, who were then contacted by phone. Patients who agreed to participate were scheduled for a baseline study visit <1 month before surgery and gave written informed consent before engaging in study activities. Study recruitment began in November 2013 and follow‐up visits were completed in December 2017.
Patient and surgical characteristics are described using mean (standard deviation) or median (interquartile range) for numeric variables and N (%) for categorical variables.
Intraoperative management
All study patients received general anesthesia with an inhaled anesthetic (i.e., isoflurane) or an intravenous propofol infusion. Anesthetic administration was titrated to maintain bispectral index processed EEG values between 40 and 60. There were no restrictions on regional or neuraxial blocks (e.g., spinal or epidural anesthesia), adjunct anesthetic drugs, or surgical technique, other than the exclusion of cardiac and neurosurgical patients.
CSF sample collection, biomarker measurements, and APOE genotyping
CSF samples were collected before and 24 h and 6 weeks after surgery using a 25‐g pencil‐point needle by aspiration with a 10‐mL polypropylene syringe, using our established protocol. 37 CSF samples were then aliquoted into Sarstedt 1.5‐mL polypropylene microcentrifuge tubes (VWR; Radnor, PA) and frozen at −80°C within 1 h. CSF samples were maintained at −80°C without any freeze/thaw cycles until they were thawed together for batched analysis. One hundred and five (95%) patients contributed CSF data to the analysis, and 86 (78%) had a complete dataset of CSF biomarker data at all three time points. CSF tau, p‐tau‐181p and Aβ42 measurements were performed using the AlzBio3 platform. 30 Multiple imputation methods were used for missing data in patients with incomplete CSF sample data. Since these biomarker data were not normally distributed, change values are reported as medians with median absolute deviations (MAD, i.e., the nonparametric equivalent of a standard deviation). CSF Aβ42 and tau results were classified as pathologic based on cutoffs of <250 and >93 pg/mL, respectively. 38 APOE genotyping was performed as described. 30
CSF biomarker metrics
We defined two change scores of interest in our analysis. Our primary biomarker change of interest was the change from baseline to 24 h and from baseline to 6 weeks in tau concentrations, defined as the 24‐h concentration minus the baseline level, and 6‐week concentration minus the baseline level. For secondary analysis, we defined the 24‐h and 6‐week CSF change scores for p‐tau‐181p, Aβ, and the ratios of tau and p‐tau‐181p to Aβ in the same way. Additionally, we considered the trajectory of CSF concentrations over time to investigate if there was change across the study period.
CSF imputation strategy
We included all patients that returned for follow‐up cognitive testing in our primary analysis cohort. One hundred and five (95%) patients contributed CSF data to the analysis, and 86 (78%) had a complete dataset at all three time points. In order to include patients with incomplete data in the analysis, we pursued multiple imputation using SRCWare software v0.2 based on all known patient and surgical factors as well as observed cognitive test scores and CSF concentrations. SRCware employs multivariate sequential regression to replace missing data within a plausible range to represent uncertainty in the true value. This yields multiple imputed datasets, which are analyzed in the same manner as the rest of the data. The analyses are then pooled to produce results that properly reflect uncertainty resulting from the missing data points. 39 We created 10 imputation datasets and used standard methods to pool across imputed sets. Sensitivity analysis was conducted on patients with complete data (i.e., excluding patients with imputed data) to ensure all conclusions were robust to this imputation technique.
Cognitive testing
Patients completed cognitive tests at both the preoperative and 6‐week postoperative study visits. Individual tests are listed in Table 2. To characterize cognitive function over time while minimizing potential redundancy in the cognitive measures, a factor analysis with oblique rotation was performed on 10 of the cognitive test scores administered in this study. Scoring coefficients (weights) of each test on each factor were determined using the rotated factor solution from the factor analysis conducted on baseline scores among 389 non‐cardiac patients in a prior study. 40 Factor scores of each subject in our cohort were computed for all time points using the same scoring coefficients, so that the cognitive domain structure remained consistent and comparable over time. Factor analysis suggested a four‐factor solution, which accounts for 87% of the variability in the original test scores and represents four cognitive domains: (1) unstructured verbal memory (i.e., the ability to remember from a narrative); (2) visual memory; (3) executive function; and (4) attention and concentration. We defined continuous cognitive outcome measures for each cognitive domain as the change in cognitive score calculated by subtracting the baseline from the follow‐up domain score (a change score of 0 indicates no change from baseline, while a negative score indicates cognitive decline, and a positive score indicates cognitive improvement). We also defined a global cognitive index outcome as the average of the four domain change scores, with global cognitive change obtained by subtracting the baseline from the follow‐up average of the four domain scores. We tabulated the cohort performance on the battery of cognitive tests administered, with mean scores, standard deviations, and score ranges at baseline and 6 weeks.
Table 2.
Cognitive assessment data.
| Cognitive domains and tests | Baseline preoperative testing | 6‐week postoperative follow‐up | Tests for change in domains |
|---|---|---|---|
| Verbal memory domain | 0.46 (0.89) | 0.12 (1.05) | 0.003 |
| Immediate RANDT gist | 7.20 (1.47) | 6.72 (1.75) | |
| 4–10 | 2–9 | ||
| Immediate RANDT verbatim | 10.54 (3.03) | 9.83 (3.39) | |
| 4–18 | 2–18 | ||
| Delay RANDT gist | 6.60 (1.86) | 6.21 (2.21) | |
| 0–10 | 0–10 | ||
| Delay RANDT verbatim | 8.92 (3.25) | 7.99 (3.47) | |
| 4–18 | 0–17 | ||
| Hopkins Verbal Learning Initial (3 Trial Sum) 1 | 23.90 (5.98) | 24.37 (5.79) | |
| 7–35 | 8–35 | ||
| Hopkins delayed recall 1 | 7.87 (3.59) | 7.94 (3.46) | |
| 0–12 | 0–12 | ||
| Hopkins delayed recognition 1 | 10.32 (1.90) | 10.08 (1.97) | |
| 3–12 | 2–12 | ||
| Visual memory domain | −0.07 (0.97) | 0.06 (0.93) | 0.060 |
| Wechsler reproduction immediate (3 trial sum) | 6.27 (2.83) | 6.85 (2.71) | |
| 0–11 | 1–11 | ||
| Wechsler reproduction delay (3 trial sum) | 5.65 (2.93) | 5.82 (2.90) | |
| 0–11 | 0–11 | ||
| Executive function domain | 0.08 (1.07) | 0.47 (1.01) | <0.001 |
| Digit symbol | 43.15 (11.74) | 46.10 (12.15) | |
| 10–69 | 0–67 | ||
| Trails Making Test Part A 1 | 45.32 (96.04) | 39.20 (42.84) | |
| 16–1028 | 16–450 | ||
| Trails Making Test Part B | 126.86 (105.17) | 98.88 (90.24) | |
| 32–600 | 26–514 | ||
| Attention/concentration domain | −0.18 (0.86) | −0.19 (0.86) | 0.822 |
| Digit span repeat forwards | 7.36 (2.03) | 7.62 (2.15) | |
| 2–12 | 3–14 | ||
| Digit span repeat backwards | 6.10 (2.34) | 5.77 (2.21) | |
| 0–14 | 1–12 | ||
| Cognitive Index Score | 0.07 (0.74) | 0.11 (0.75) | 0.175 |
Values are given as mean (SD). One hundred and ten patients completed each test at each time point, with the following exceptions due to test administration deviations or the inability of some patients to complete certain tests: Hopkins Delayed Recognition (N = 109 at pre‐operative testing), Digit Symbol (N = 109 at preoperative testing), and Trails Making test Part B (N = 107 at preoperative testing, N = 108 at 6‐week postoperative testing). The domain and cognitive index scores are based on the factor solution from a previous study by McDonagh et al and include multiply imputed values for the four patients with unobserved test values.
Not included in the prior study test battery from which factor weights were derived, so not included in cognitive factor analysis.
Analysis of association between cognitive change and CSF biomarker change
All patients who returned for 6‐week postoperative cognitive testing were included in the primary analysis. To investigate the association of preoperative to 6‐week postoperative changes (a subtraction of baseline from follow‐up) in CSF biomarkers and cognition, we used Spearman’s correlation coefficients (r s), as postoperative CSF biomarker data were not normally distributed. 30 We used multivariable analyses to account for potential confounders of relationships between postoperative changes in CSF biomarkers and cognition, including age, years of education, baseline cognitive index, and APOE4 allele carrier status. To assess CSF biomarker changes over time, we used Friedman's test. Analyses were performed in SAS v9.4 (SAS Inc., Cary, NC, USA). Values of p are reported with a Benjamini–Hochberg false discovery rate correction at α = 0.05, unless otherwise indicated. 41
Sample size and power analysis
Based on prior work, 30 we calculated that N = 108 patients would provide >80% power to detect a Spearman correlation of ≥0.3 between preoperative to 6‐week postoperative changes in the continuous cognitive index score and CSF tau level changes. We enrolled 140 patients to ensure that ≥108 patients would return for 6‐week postoperative follow‐up.
Results
Patient demographics and baseline neurocognitive and intraoperative characteristics
Study subject enrollment is depicted in Figure 1; baseline and intraoperative characteristics are listed in Table 1. The mean age was ~69 years (SD = 6.5 years). One patient had a clinical diagnosis of mild cognitive impairment; none of the patients had a clinical diagnosis of AD or related dementias. Consistent with this, the median preoperative Mini‐Mental State Examination score was 29, and ~94% of patients had a score of 25 or higher. Over 95% of patients underwent thoracic, orthopedic, ENT, urologic, or general surgery; typical surgery duration was roughly 2 h. Most patients (>85%) did not receive an epidural or peripheral nerve block. Roughly half of the patients received inhaled anesthesia with isoflurane, and roughly half received propofol‐based total intravenous anesthesia. Based on NIA‐AA AD stage classifications 42 , 43 , 44 and AD pathology threshold values, 38 ~83% (N = 91) of this cohort would be classified as Aβ−tau− (i.e., normal), ~14% (N = 15) would be Aβ+tau− (i.e., likely preclinical AD), ~1% (N = 1) would be Aβ−tau+ (suspected non‐AD pathophysiology), and ~3% (N = 3) would be classified as Aβ+tau+ (likely either preclinical AD, MCI, or dementia due to AD; percentages add to more than 100 due to rounding).
Figure 1.
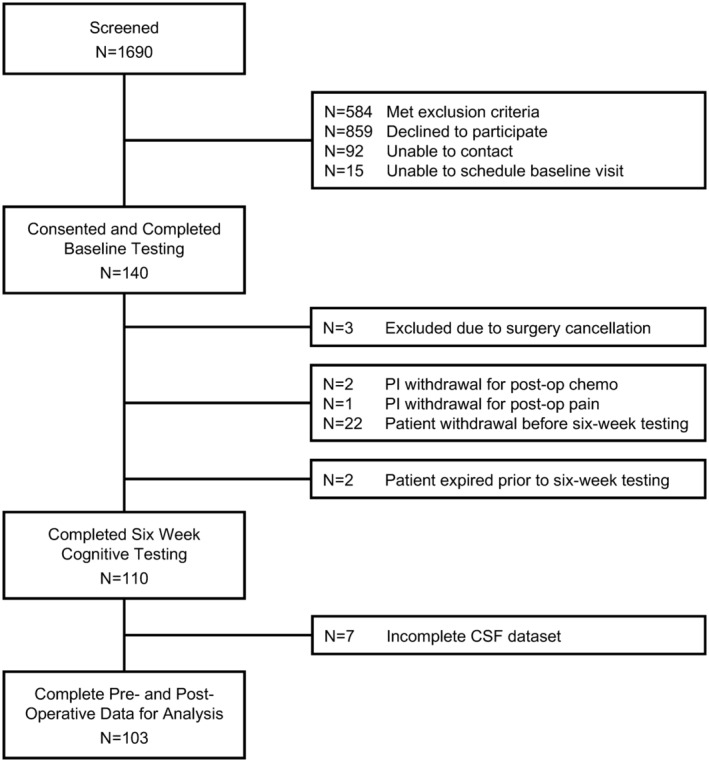
MADCO‐PC study flow diagram. Out of 140 patients who consented to and enrolled in the study and underwent baseline testing, 110 patients returned for cognitive testing at 6 weeks after surgery. These 110 patients were used in the final data analysis.
Table 1.
Baseline demographic and intraoperative characteristics.
| Total (N = 110) | |
|---|---|
| Age (years) (SD) | 69.2 (6.5) |
| Race (%) | |
| Black or African American | 10 (9.1) |
| Caucasian/White | 99 (90.0) |
| Not reported/declined | 1 (0.9) |
| Gender (male) (%) | 69 (62.7) |
| Height (cm) (Q1, Q3) | 171.9 (10.7) |
| Weight (kg) (SD) | 85.7 (18.8) |
| BMI (kg/m2) [Q1, Q3] | 28.6 [24.5, 32.0] |
| Years of education [Q1, Q3] | 16.0 [12.0, 18.0] |
| MMSE total score [Q1, Q3] | 29.0 [28.0, 29.0] |
| MMSE category (%) | |
| <20 | 1 (0.9) |
| 20–24 | 6 (5.5) |
| 25–30 | 103 (93.6) |
| Mean Baseline Cognitive Index (SD) | 0.074 (0.74) |
| Median baseline Aβ (MAD) | 360.1 (51.4) |
| Median baseline p‐tau (MAD) | 27.0 (6.2) |
| Median baseline p‐tau/Aβ (MAD) | 0.076 (0.018) |
| Median baseline tau (MAD) | 47.6 (8.2) |
| Median baseline tau/Aβ (MAD) | 0.135 (0.026) |
| APOE4 positive | 35 (32.4) |
| ASA physical status classification (%) | |
| 1 | 1 (0.9) |
| 2 | 23 (20.9) |
| 3 | 84 (76.4) |
| 4 | 2 (1.8) |
| Surgery type (%) | |
| Thoracic | 11 (10.0) |
| General | 31 (28.2) |
| Gynecologic | 2 (1.8) |
| Orthopedic | 21 (19.1) |
| Otolaryngology head and neck | 2 (1.8) |
| Plastic | 3 (2.7) |
| Urologic | 40 (36.4) |
| Nerve block type (%) | |
| None | 94 (85.5) |
| Peripheral nerve block | 15 (13.6) |
| Epidural block | 1 (0.9) |
| Surgery duration (minute) [Q1, Q3] | 140.0 [100.0, 199.0] |
| Case average BIS [Q1, Q3] 1 | 46.1 [41.9, 51.5] |
| Received inhaled anesthetic for >50% of case | 56 (50.9) |
| Case average aaMAC [Q1, Q3] 2 | 0.7 [0.7, 0.8] |
| aaMAC hours [Q1, Q3] 2 | 1.6 [1.3, 2.7] |
| Received intraoperative dexmedetomidine (%) | 13 (11.8) |
| Received intraoperative ketamine (%) | 23 (20.9) |
| Received intraoperative propofol >400 mg (%) | 55 (50.0) |
| Intraoperative propofol dose (mg) (Q1, Q3) 3 | 1357.0 [975.5, 2536.8] |
| Hospital LOS (days) [Q1, Q3] | 2.0 [1.0, 3.0] |
aaMAC, age‐adjusted minimum alveolar concentration for inhaled anesthetics; BIS, Bispectral Index (processed EEG value); LOS, length of stay; MAD, median absolute deviation; MMSE, Mini‐Mental State Examination; SD, standard deviation.
Among those who had BIS values for >50% of the case.
Among those who received inhaled anesthetic for >50% of the case.
Among those who received >400 mg intraoperative propofol.
Pre‐ to postoperative CSF biomarker trajectories
No significant changes were found in CSF tau or Aβ levels from before to 24 h (median [MAD] tau change: 0.00 [4.36] pg/mL, p = 0.853; Aβ: −0.88 [22.75] pg/mL, p = 0.871) or from before to 6 weeks (−1.21 [3.98] pg/mL, p = 0.827; Aβ: −5.09 [24.40] pg/mL, p = 0.827) after surgery (Fig. 2). There was also no significant change in the tau/Aβ ratio from before to 24 h after anesthesia (median [MAD] tau/Aβ ratio: −0.001 [0.018], p = 0.864) or from before to 6 weeks after anesthesia (0.001 [0.014], p = 0.879; Fig. 2).
Figure 2.
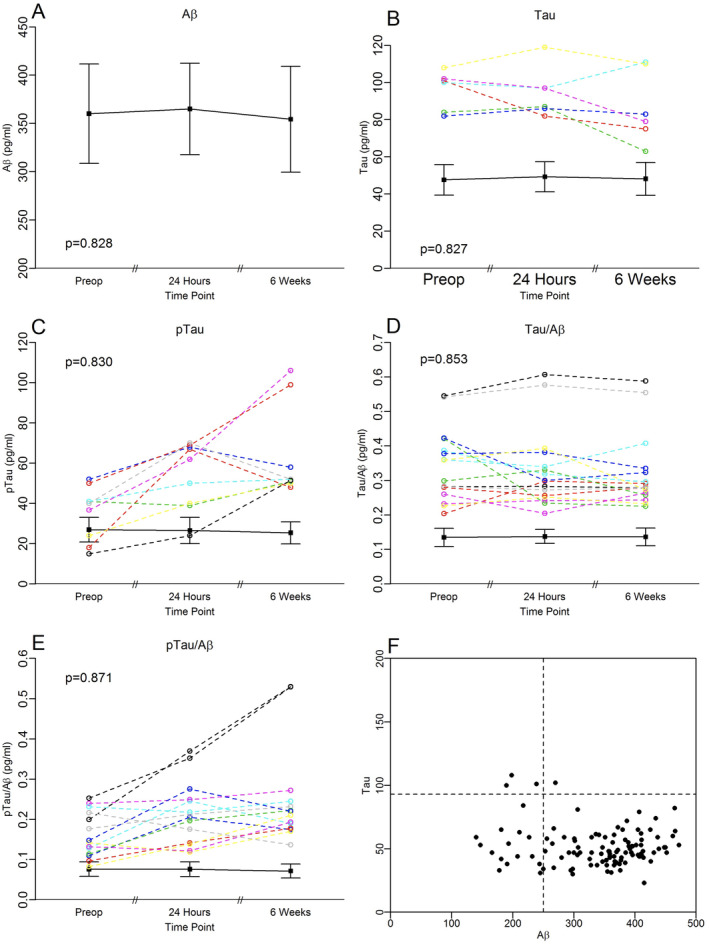
CSF biomarkers in MADCO‐PC surgical patients before, 24 h after, and 6 weeks after anesthesia and surgery. In (A–F) black lines represent the group medians, error bars represent MADs. Colored lines in (A–E) represent biomarker trajectories for individual patients who had values >1.5 the interquartile range from the median (a standard statistical definition for outliers 75 ) at any time point. (A) CSF Aβ levels. CSF Aβ42 levels were within 1.5 times the IQR from the group median at all three time points in all patients. (B) CSF tau levels. Six patients had CSF tau levels >1.5 times the IQR from the group median at one or more time points. (C) CSF p‐tau‐181p levels. Nine patients had CSF p‐tau‐181p levels >1.5 times the IQR from the group median at one or more time points. In one of the two patients with p‐tau‐181p levels >90 at the 6‐week time point, CSF samples were unavailable at the preoperative and 24 h time points, so these values were imputed (see Methods section). (D) CSF tau/Aβ ratio. Sixteen patients had CSF tau/Aβ42 ratios >1.5 times the IQR from the group median at one or more time points. (E) CSF p‐tau‐181p/Aβ ratio. 14 patients had CSF p‐tau‐181p/Aβ42 ratios >1.5 times the IQR from the group median at one or more time points. In one of the two patients with a p‐tau‐181p/Aβ ratio >0.5 at the 6‐week time point, CSF samples were unavailable at the preoperative and 24 h time points, so these values were imputed (see Methods section). (F) CSF tau versus Aβ plot in MADCO‐PC patients, with dashed lines representing the cutoffs for pathological Aβ and tau levels. [Colour figure can be viewed at wileyonlinelibrary.com]
Similarly, there was no significant group change in CSF p‐tau‐181p from before to 24 h after surgery (median [MAD] p‐tau‐181p change: 1.10 [5.01] pg/mL, p = 0.771) or from before to 6 weeks after surgery (−0.44 [5.90] pg/mL, p = 0.871; Fig. 2). All nine patients had CSF p‐tau‐181p levels that were >1.5 times the interquartile range at one or more time points (Fig. 2C). All these nine patients had a CSF p‐tau‐181p increase from before to 6 weeks after surgery, and two of them had a ~2‐ to 3‐fold increase in CSF p‐tau‐181p over this interval. At a group level, there was no significant change in the p‐tau‐181p/Aβ ratio from before to 24 h after anesthesia (median [MAD] p‐tau‐181p/Aβ ratio change: 0.002 [0.014], p = 0.814) or from before to 6 weeks after anesthesia (0.002 [0.018], p = 0.830; Fig. 2). Yet, 14 patients had a p‐tau‐181p/Aβ ratio >1.5 times the interquartile range at one or more time points (Fig. 2E), including two patients whose p‐tau‐181p/Aβ ratios increased by ~2‐fold from before to 6 weeks after surgery—the same two patients who had a ~2‐ to 3‐fold increase in p‐tau‐181p levels over this interval in Figure 2C. Additionally, we observed no effect of surgery type (i.e., urologic, general/abdominal, thoracic, orthopedic, or other) on preoperative to 6‐week postoperative change in CSF tau, p‐tau‐181p or Aβ levels or the p‐tau‐181p/Aβ or tau/Aβ ratios (data not shown).
Pre‐ to postoperative cognitive trajectories
Cognitive domain and overall cognitive index scores before and 6 weeks after surgery are shown in Figure 3. There was a significant postoperative decline in verbal memory across all patients (mean [95% CI]: −0.346 [−0.523, −0.170], p = 0.003). Yet, there was a significant improvement in executive function from before to 6 weeks after surgery (Table 2; mean [95% CI]: 0.394 [0.310, 0.479], p = <0.001). There was no significant change in attention/concentration (Table 2; mean [95% CI]: −0.012 [−0.114, 0.090], p = 0.822) or in visual memory (Table 2; mean [95% CI]: 0.123 [0.003, 0.244], p = 0.060). There was no significant change in overall cognitive index (the average of the four cognitive domain scores) from before to 6 weeks after surgery (mean [95% CI]: 0.040 [−0.018, 0.098], p = 0.175). There was no difference in overall cognitive index change from before to 6 weeks after surgery as a function of surgery type (i.e., orthopedic, thoracic, general or other), or as a function of surgery duration (Table 4). Approximately 27% of patients had a ≥1 standard deviation decline and 26% had a ≥1 standard deviation increase in at least one cognitive domain from before to after surgery.
Figure 3.
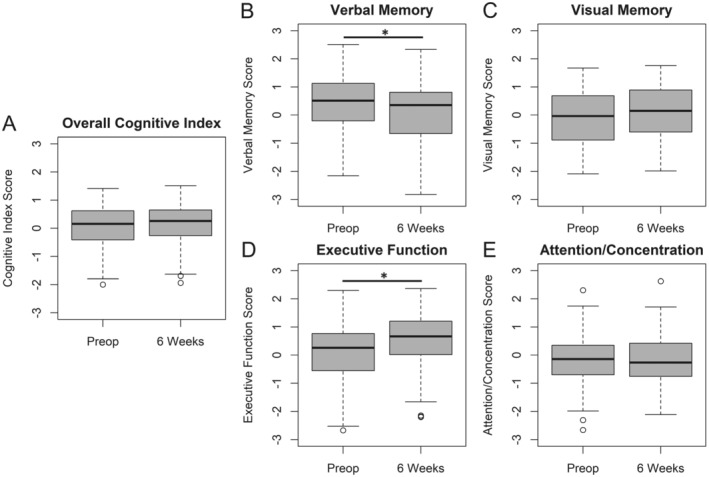
Cognitive results before and 6 weeks after surgery. (A) overall cognitive index; (B) verbal memory; (C) visual memory; (D) executive function; (E) attention/concentration. A positive score on the y‐axis represents an improvement in cognitive score from before to after surgery, and vice versa. * indicates a significant difference between the preoperative and 6‐week timepoints at p < 0.05.
Table 4.
Multivariable linear regression models for the association of tau or tau/β change from preop to either 24 h or 6 weeks after surgery, with cognitive change from preop to 6 weeks after surgery.
| Parameter | Estimate (95% CI) | p‐value |
|---|---|---|
| Model 1—Association of preop to 24‐h CSF tau change, preop to 6‐week postop cognitive change | ||
| Intercept | 0.37 (−0.35, 1.1) | 0.307 |
| Baseline Cognitive Index | −0.14 (−0.24, −0.05) | 0.003 |
| Age (per year) | −0.01 (−0.02, 0.00) | 0.072 |
| Years of education | 0.02 (0.00, 0.04) | 0.100 |
| APOE4 carrier versus non‐carrier | −0.05 (−0.17, 0.08) | 0.465 |
| Surgery duration (per 30 min) | 0.01 (−0.01, 0.02) | 0.348 |
| Tau change 24 h | 0.00 (−0.01, 0.01) | 0.521 |
| Model 2—Association of preop to 24‐h CSF tau/Aβ change, preop to 6‐week postop cognitive change | ||
| Intercept | 0.36 (−0.36, 1.09) | 0.324 |
| Baseline Cognitive Index | −0.14 (−0.24, −0.05) | 0.004 |
| Age (per year) | −0.01 (−0.02, 0.00) | 0.078 |
| Years of education | 0.02 (0.00, 0.04) | 0.010 |
| APOE4 carrier versus non‐carrier | −0.04 (−0.17, 0.08) | 0.474 |
| Surgery duration (per 30 min) | 0.01 (−0.01, 0.03) | 0.326 |
| Tau/Aβ change 24 h | −0.01 (−1.6, 1.59) | 0.995 |
| Model 3—Association of preop to 6‐week postop CSF tau change, preop to 6‐week postop cognitive change | ||
| Intercept | 0.36 (−0.37, 1.08) | 0.330 |
| Baseline Cognitive Index | −0.14 (−0.24, −0.05) | 0.004 |
| Age (per year) | −0.01 (−0.02, 0.00) | 0.079 |
| Years of education | 0.02 (0.00, 0.04) | 0.101 |
| APOE4 carrier versus non‐carrier | −0.04 (−0.17, 0.08) | 0.505 |
| Surgery duration (per 30 min) | 0.01 (−0.01, 0.02) | 0.334 |
| Tau change 6‐week | 0.00 (−0.01, 0.01) | 0.799 |
| Model 4—Association of preop to 6‐week postop CSF tau/Aβ change, preop to 6‐week postop cognitive change | ||
| Intercept | 0.36 (−0.36, 1.09) | 0.321 |
| Baseline Cognitive Index | −0.14 (−0.24, −0.05) | 0.004 |
| Age (per year) | −0.01 (−0.02, 0) | 0.075 |
| Years of education | 0.02 (0, 0.04) | 0.094 |
| APOE4 carrier versus non‐carrier | −0.05 (−0.17, 0.08) | 0.442 |
| Surgery duration (per 30 min) | 0.01 (−0.01, 0.02) | 0.323 |
| Tau/ Aβ change 6‐week | 0.36 (−1.32, 2.05) | 0.667 |
p‐values are uncorrected for multiple comparisons.
Relationships between pre‐ to postoperative changes in CSF biomarkers and cognition
Next, we evaluated potential associations between individual patient CSF biomarker (Fig. 2) and cognitive trajectories (Fig. 3) from before to 6 weeks after anesthesia/surgery. No correlation was observed between the continuous cognitive change index and 24‐h or 6‐week changes in CSF tau (24‐h change: r s = −0.01, 95% CI = [−0.21, 0.19], p = 0.966; 6‐week change: r s = −0.03, 95% CI = [−0.23, 0.18], p = 0.879; or CSF Aβ (24‐h change: r s = 0.03, 95% CI = [−0.17, 0.24], p = 0.871; 6‐week change: r s = −0.08, 95% CI = [−0.29, 0.13], p = 0.830). There was also no significant correlation between the continuous cognitive change index and 24‐h or 6‐week changes in CSF tau/Aβ (24‐h change: r s = −0.05, 95% CI = [−0.25, 0.15], p = 0.853; 6‐week change: r s = 0.02, 95% CI = [−0.19, 0.22], p = 0.902). Similarly, there was no significant correlation between the continuous cognitive change index and 24‐h or 6‐week change in CSF p‐tau‐181p levels (24‐h change: r s = 0.16, 95% CI = [−0.05, 0.37], p = 0.721; 6‐week change: r s = 0.12, 95% CI = [−0.08, 0.32], p = 0.814) or the CSF p‐tau‐181p/Aβ ratio (24‐h change: r s = 0.12, 95% CI = [−0.10, 0.33], p = 0.827; 6‐week change: r s = 0.16, 95% CI = [−0.05, 0.34], p = 0.721) after anesthesia/surgery (Fig. 4). There were also no significant correlations between changes from before to either 24 h or 6 weeks after surgery in any of the aforementioned CSF biomarkers, and changes from before to 6 weeks after surgery in any of the four individual cognitive domains (Table 3). There were no significant differences in the change in any of these CSF biomarkers from before to either 24 h or 6 weeks after surgery between patients who did versus those who did not have a ≥1 SD decrease in ≥1 cognitive domains from before to 6 weeks after surgery (p > 0.05 for each).
Figure 4.
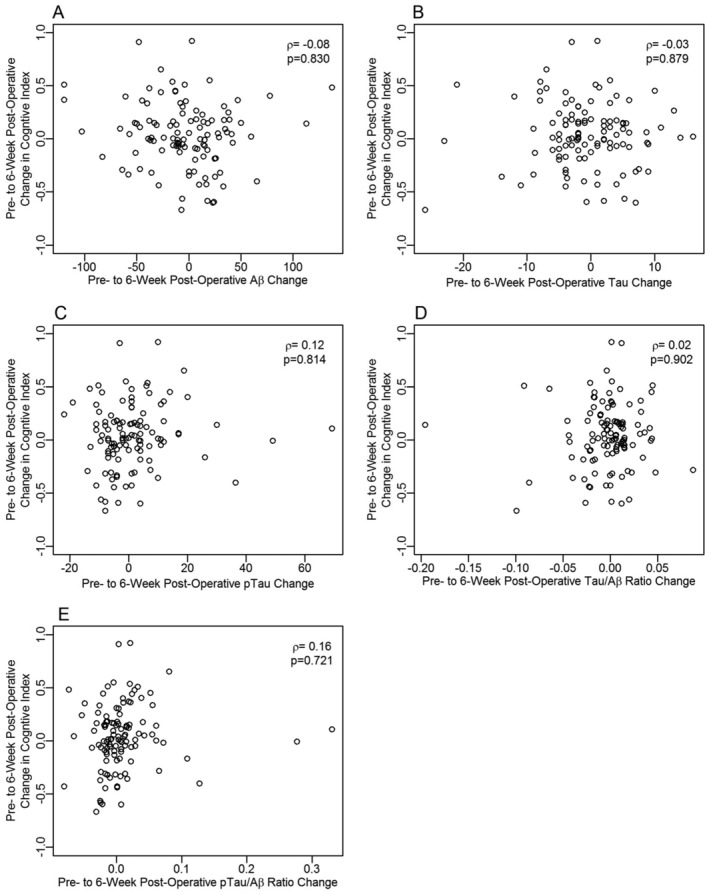
Correlation between changes in CSF biomarkers and overall cognitive index change from before to 6 weeks after surgery and anesthesia. (A) CSF Aβ; (B) CSF tau; (C) CSF p‐tau‐181p; (D) tau/Aβ ratio; (E) p‐tau‐181p/Aβ ratio. A positive score on the x‐ or y‐axis represents an improvement in cognitive score or an increase in the indicated biomarker from before to after surgery, respectively, and vice versa.
Table 3.
Correlations between preoperative to 24‐h postoperative CSF biomarker changes and preoperative to 6‐week postoperative across cognitive domain score change.
| Verbal domain | Figural domain | Attention/concentration | Executive function | |||||
|---|---|---|---|---|---|---|---|---|
| Spearman’s rho | p‐value | Spearman’s rho | p‐value | Spearman’s rho | p‐value | Spearman’s rho | p‐value | |
| 24‐h CSF change | ||||||||
| Aβ | 0.04 (−0.16, 0.24) | 0.853 | −0.07 (−0.30, 0.17) | 0.853 | 0.06 (−0.15, 0.27) | 0.853 | −0.08 (−0.28, 0.13) | 0.830 |
| p‐Tau | 0.13 (−0.07, 0.31) | 0.814 | 0.10 (−0.11, 0.31) | 0.827 | −0.10 (−0.30, 0.11) | 0.827 | 0.06 (−0.16, 0.27) | 0.853 |
| Tau | −0.05 (−0.25, 0.16) | 0.853 | 0.05 (−0.16, 0.26) | 0.853 | −0.03 (−0.26, 0.19) | 0.871 | 0.05 (−0.16, 0.26) | 0.853 |
| p‐Tau/Aβ | 0.10 (−0.11, 0.29) | 0.827 | 0.12 (−0.10, 0.32) | 0.827 | −0.10 (−0.29, 0.10) | 0.827 | 0.05 (−0.17, 0.27) | 0.853 |
| Tau/Aβ | −0.08 (−0.28, 0.12) | 0.83 | 0.12 (−0.08, 0.31) | 0.814 | −0.06 (−0.28, 0.16) | 0.853 | 0.05 (−0.17, 0.26) | 0.853 |
| 6‐week CSF change | ||||||||
| Aβ | −0.09 (−0.29, 0.12) | 0.830 | −0.01 (−0.22, 0.21) | 0.981 | 0.10 (−0.11, 0.30) | 0.827 | −0.13 (−0.32, 0.08) | 0.814 |
| p‐Tau | 0.11 (−0.09, 0.31) | 0.827 | 0.09 (−0.13, 0.30) | 0.83 | 0.08 (−0.12, 0.28) | 0.830 | 0.02 (−0.18, 0.21) | 0.902 |
| Tau | −0.13 (−0.33, 0.08) | 0.814 | 0.11 (−0.10, 0.32) | 0.827 | 0.00 (−0.20, 0.21) | 0.992 | −0.00 (−0.21, 0.20) | 0.992 |
| p‐Tau/Aβ | 0.15 (−0.06, 0.34) | 0.792 | 0.06 (−0.15, 0.26) | 0.853 | 0.06 (−0.13, 0.25) | 0.851 | 0.06 (−0.14, 0.26) | 0.853 |
| Tau/Aβ | −0.04 (−0.24, 0.16) | 0.864 | 0.05 (−0.15, 0.24) | 0.853 | −0.07 (−0.26, 0.14) | 0.851 | 0.08 (−0.15, 0.29) | 0.841 |
In multivariable models adjusting for age, years of education, APOE4 genotype, and baseline preoperative cognitive index, there were no significant associations between postoperative changes in tau (24‐h change: =0.003, 95% CI = [−0.005, 0.011], p = 0.483; 6‐week change: = − 0.001, 95% CI = [−0.010, 0.007], p = 0.738) or tau/Aβ (24‐h change: =0.054, 95% CI = [−1.547, 1.656], p = 0.946; 6‐week change: =0.381, 95% CI = [−1.295, 2.056], p = 0.652) and 6‐week postoperative cognitive change (Table 4). Similarly, in a model containing surgery type, there was also no significant relationship between 6‐week postoperative changes in tau and cognition, nor was there any association between surgery type and 6‐week postoperative cognitive change. There was also no correlation between postoperative changes in overall cognitive index and CSF tau levels or the tau/Aβ ratio in a sensitivity analysis excluding imputed data, p > 0.05 for each comparison).
Discussion
These data demonstrate no significant association between preoperative to 24‐h or 6‐week postoperative changes in CSF tau (or p‐tau‐181p, Aβ, and the tau/Aβ and p‐tau‐181p/Aβ ratios) and cognitive change from before to 6 weeks after non‐cardiac, non‐neurologic surgery in older adults. Most patients showed no clinically significant change in CSF Aβ, tau, or p‐tau‐181p levels from before to after surgery. 45 There was no change in overall cognition from before to 6 weeks after surgery, though this was likely due to opposing decreases in verbal memory and increases in executive function.
The finding of no significant group‐level changes in CSF tau, p‐tau‐181p, or Aβ levels or their ratios from before to 24 h or to 6 weeks after surgery contrasts with prior reports from both our group and others of significant 24‐h postoperative increases in CSF and plasma tau levels. 29 , 30 , 46 , 47 These differences are notable given that CSF biomarker measurements in these prior studies were obtained using the same assay as used here for CSF biomarkers, in the same laboratory used here. 29 , 30 However, all patients in these prior CSF studies underwent neurosurgical or ENT procedures that involved manipulation of their brain and/or dura, 29 , 30 whereas none of the patients in this study did. Another recent report found that CSF tau levels increased linearly from before to 48 h after surgery, although this study obtained CSF via intrathecal catheters. 48 Intrathecal catheters themselves can cause neuroinflammation and neurologic symptoms. 49 , 50 , 51 Thus, the most parsimonious interpretation of these data is that while surgery on the central nervous system leads to CSF tau increases 24 h later, non‐neurologic surgery and general anesthesia are not associated with a significant change in CSF biomarkers of amyloid or tau pathology 1 day or 6 weeks later in most older adults.
The lack of postoperative change in CSF Aβ and tau levels reported here also differ with data from mouse models 22 , 23 , 24 and in vitro 25 , 26 systems demonstrating that anesthetic drugs and surgical stress promote Aβ and tau pathology. The discrepancy between these human data versus basic laboratory studies may be explained by species differences between humans and mice, or by differences in physiologic management between human anesthetic cases versus mouse studies, as many of these mouse model studies did not report intraoperative blood pressure, heart rate, oxygenation, and/or temperature data. 23 , 24 , 25 Further, Aβ, tau, and p‐tau‐181p levels were measured here in human CSF, whereas many rodent studies examined these proteins in brain tissue sections 22 , 23 , 24 ; the differing results could reflect differences in the specimen types studied (i.e., human CSF versus mouse brain tissue).
The postoperative cognitive change data are reported here as group means and 95% confidence intervals. Most patients had a decrease in verbal memory from before to 6 weeks after surgery, yet an improvement in executive function over this period. These data are consistent with prior work demonstrating that most patients show improved executive function at 6 weeks after surgery. 12 To our knowledge, prior studies have not shown a verbal memory decline in the majority of older adults at 6 weeks after major surgery, although many prior studies simply reported the number or percentage of patients who met a specific decline threshold rather than providing mean verbal memory score values across all patients before and after surgery. 12 , 52 Overall, since the average patient in this study had no significant change in overall cognitive function from before to 6 weeks after surgery, the cognitive data presented here offer a reassuring conclusion to older surgical patients.
Although average cognitive index and CSF biomarker levels did not significantly change from before to 6 weeks after surgery, there was significant variation among individual patients in these measures over time. This wide variation in postoperative changes in cognition and CSF biomarker levels among individual patients thus emphasizes the question this study was designed to address: Is there a relationship between postoperative changes in CSF biomarkers associated with AD and postoperative changes in cognition?
At a group level, this study provides a clear negative answer: there was no significant correlation between postoperative changes in overall cognition and CSF Aβ, tau, or p‐tau‐181p levels or their ratios (Fig. 4). Further, aside from the lack of statistical significance, the relatively small correlation coefficient values observed between postoperative changes in cognition and CSF biomarkers strongly suggest that even a much larger study would be unlikely to find a significant or clinically meaningful correlation between these variables.
This study has several limitations. First, the vast majority of the patients in this study were cognitively normal (median Mini‐Mental State Examination [MMSE] = 29), none had a clinical diagnosis of AD or other dementia, only one had a diagnosis of MCI, only 16 had low CSF Aβ or high CSF tau levels (i.e., A + T− or A−T+), and only three patients had low CSF Aβ and high CSF p‐tau‐181p levels (i.e., A + T+). Thus, these data may have limited generalizability to older surgical patients with baseline amyloid and tau pathology, and those with baseline dementia or MCI, who may be at higher risk of cognitive decline after surgery. 20 The extent to which anesthesia and surgery accelerate amyloid and/or tau pathology and/or cognitive decline in patients who already have significant levels of amyloid and tau pathology remains an important question for future study.
Second, these data do not show a correlation between AD‐related CSF biomarker change and cognitive change from before to 6 weeks after surgery, a time interval during which cognitive deficits have been detected after anesthesia and surgery in numerous prior studies. 10 , 11 , 12 , 16 , 17 , 40 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 Yet, this does not exclude the possibility that such a correlation exists over a longer postoperative follow‐up period. However, with longer time intervals postsurgery, it becomes less clear that any changes in CSF biomarkers or cognition are directly related to the surgery; this is partly why the perioperative neurocognitive disorders nomenclature 11 does not use the modifier “perioperative” for cognitive dysfunction occurring >1‐year postsurgery. Nonetheless, since cognitive decline in AD typically occurs over 8–10 years, 71 future studies should examine whether there are correlations between cognitive and CSF AD‐related biomarker changes over longer periods after surgery.
Third, 30 of the original 140 patients enrolled were lost to follow‐up prior to the 6‐week follow‐up visit. This rate of loss to follow‐up is not surprising in studies like this that involve invasive procedures in older patients following major surgery. Yet, the loss to follow‐up was not entirely random; patients lost to follow‐up were significantly more likely to be African American, a challenge that other aging researchers have described and for which new strategies are being discussed to improve equity in subject representation for future studies. 72 , 73 Patients lost to follow‐up were also more likely to have a lower baseline cognitive index value, and had slightly lower aaMAC values (~0.08 aaMAC units lower, p = 0.0086; Table S1). Nonetheless, with 110 patients who returned for 6‐week follow‐up, this study still had >80% power to detect a Spearman r s ≥ 0.3 for the correlation between postoperative CSF tau change and preoperative to 6‐week postoperative cognitive change, and no such correlation was seen. Further, a group of patients with complete 6‐week follow‐up data that matched key demographics (race, baseline cognition, gender, and surgical aaMAC) of the 30 patients lost to follow‐up also showed no significant correlations between postoperative changes in CSF AD‐related biomarkers and cognition (Tables S2–S5). Thus, even if the patients lost to follow‐up had returned for the 6‐week postoperative visit and contributed data to the study, it would still be highly unlikely that there would have been a significant correlation between postoperative CSF AD‐related biomarker and cognitive changes.
Fourth, the cognitive data were not adjusted for practice or learning effects by comparison to a nonsurgical control group using reliable change index (RCI) or other regression methods. 74 We did not analyze the data this way, because our goal was simply to examine potential correlations between changes in CSF biomarkers and cognition from before to after surgery. The purpose of this study was not to determine how much of the cognitive change in individual patients was due to anesthesia/surgery versus practice or learning effects, or other potential causes of cognitive change after anesthesia/surgery (i.e., sleep disruption, pain, changes in ambulation, etc.).
To our knowledge, this study represents the largest cohort of older surgical patients to undergo cognitive testing and CSF biomarker measurements before and after surgery. The lack of significant changes in CSF Aβ, tau, and p‐tau‐181p levels after surgery observed here suggest that the wide variety of non‐cardiac, non‐neurologic surgical procedures and anesthetic techniques studied here are unlikely to cause a significant acceleration of amyloid or tau neuropathology in the vast majority of community dwelling, cognitively normal older adults. The lack of association between postoperative changes in cognition and CSF biomarkers observed here also suggests that postoperative changes in cognition are unlikely to be related to changes in Aβ or tau pathology within 1–2 months after non‐cardiac, non‐neurologic surgery in cognitively older adults. Taken together, these results should thus provide reassurance that anesthesia and surgery are unlikely to accelerate amyloid or tau related neuropathological processes associated with AD in the 6 weeks after surgery in community‐dwelling older adults.
Author Contributions
M. B., J. N. B., M. C. W., C. N., M. R., and J. P. M. contributed to writing of the manuscript. M. C. W. and M. R. performed statistical analysis. All authors contributed to analyzing and interpreting the data. M. B., B. J. C., M. J. D., E. W. M., J. W. M., B. O., D. T. L., L. M. S., H. E. W. and H. J. C. contributed to patient recruitment, study design. M. B. obtained funding for this work with input from J. P. M., J. N. B., M. C. W., H. E. W., and H. J. C.
Conflicts of Interest
M. B. has received material support (i.e., EEG monitor loan) for a postoperative recovery study in older adults from Masimo, and legal consulting fees related to postoperative neurocognitive function in older adults. J. N. B. acknowledges funding from Claret Medical, Inc. D. T. L. is an officer of AegisCN, which is developing the ApoE mimetic peptide CN‐105 for clinical use. The other authors have no other conflicts of interest to disclose related to this manuscript.
Appendix
The MADCO‐PC (Markers of Alzheimer's Disease and neuroCognitive Outcomes after Perioperative Care) Investigators also include: C. L. Amundsen, R. Beach, E. Bennett, D. G. Blazer, M. P. Bolognesi, R. Brassard, B. E. Brigman, T. Bunning, J. Carter, J. Chapman, V. Cheong, T. A. D'Amico, J. K. DeOrio, T. Ellett, D. Erdmann, R. M. Esclamado, M. N. Ferrandino, B. Funk, J. C. Gadsden, S. Grant, G. E. Garrigues, J. R. Guercio, A. S. Habib, R. K. Hallows, D. H. Harpole, M. G. Hartwig, S. T. Hollenbeck, J. Hu, R. Huang, E. Iboaya, B. A. Inman, D. W. Jang, J. Kaisen, A. Khan, S. Lagoo‐Deenadayalan, P. S. Lee, W. T. Lee, J. Lemm, H. Levinson, M. E. Lipkin, C. R. Mantyh, K. T. Martucci, D. L. McDonagh, J. Migaly, S. K. Mithani, P. Mosca, M. F. Newman, K. Ni, T. Novick, T. N. Pappas, A. N. Perez, A. C. Peterson, T. J. Polascik, A. Podgoreanu, G. M. Preminger, Q. J. Quinones, E. N. Rampersaud, A. Ray, K. Roberts, C. N. Robertson, S. Roman, S. Runyon, A. Sandler, F. Sbahi, C. D. Scales, R. P. Scheri, S. K. Smith, L. Talbot, J. K. M. Thacker, J. Thomas, B. C. Tong, Y. Toulgoat‐Dubois, A. Tu, S. N. Vaslef, N. Waldron, D. S. Warner, X. Wang, S. S. Wellman, T. Wickenheisser, M. Woldorff, C. Young, S. Zani.
Supporting information
Table S1. Demographics of lost to follow‐up (LTFU) cohort compared to returning cohort at 6 weeks.
Table S2. Comparison of cognitive trajectories between entire cohort who returned for 6‐week follow‐up, and a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.
Table S3. Correlations between preoperative CSF biomarker levels and preoperative to 6‐week postoperative cognitive change, in the entire cohort who returned for 6‐week follow‐up, and in a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.
Table S4. Correlations between preoperative to 24‐h postoperative CSF biomarker change and preoperative to 6‐week postoperative cognitive change, in the entire cohort who returned for 6‐week follow‐up, and in a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.
Table S5. Correlations between preoperative to 6‐week postoperative CSF biomarker changes and preoperative to 6‐week postoperative cognitive change, in the entire cohort who returned for 6‐week follow‐up, and in a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.
Acknowledgments
We thank Naraida Balajonda, Tiffany Bissanar, Karen Clemmons, John Encarnacion, Shahrukh Bengali, Faris Sbahi and Tressa Ellet for assistance, and Kathy Gage and Donna Crabtree for comments on this manuscript. Funding for this study was provided by the Duke Department of Anesthesiology, an International Anesthesia Research Society (IARS) Mentored Research Award (M. B.), NIH R03 AG050918 (M. B.), NIH T32 grant #GM08600, and a Jahnigen Scholars Fellowship award from the American Geriatrics Society and the Foundation for Anesthesia Education and Research (M. B.). M. B. also acknowledges additional support from NIH P30‐AG028716 and K76‐AG057022, and J. N. B. acknowledges additional support from NIH U01‐HL088942, R01‐HL130443, and R01‐AG042599. H. E. W. acknowledges support from NIH UH2 AG056925‐02 and UL1TR002553‐02. J. P. M. acknowledges support from NIH R01‐HL130443. This paper is dedicated to the memory of Dr. David S. Warner.
Funding Information
Funding for this study was provided by the Duke Department of Anesthesiology, an International Anesthesia Research Society (IARS) Mentored Research Award (M. B.), National Institutes of Health R03 AG050918 (M. B.), NIH T32 grant #GM08600, and a Jahnigen Scholars Fellowship award from the American Geriatrics Society and the Foundation for Anesthesia Education and Research (M. B.). M. B. also acknowledges additional support from NIH P30‐AG028716 and K76‐AG057022, and J. N. B. acknowledges additional support from NIH U01‐HL088942, R01‐HL130443, and R01‐AG042599. H. E. W. acknowledges support from NIH UH2 AG056925‐02 and UL1TR002553‐02. J. P. M. acknowledges support from NIH R01‐HL130443.
Funding Statement
This work was funded by American Geriatrics Society grant Jahnigen Scholars Fellowship; Foundation for Anesthesia Education and Research ; NIH grants UL1TR002553‐02, UH2 AG056925‐02, R01‐AG042599, R01‐HL130443, U01‐HL088942, K76‐AG057022, P30‐AG028716, GM08600, and R03 AG050918; International Anesthesia Research Society (IARS) Mentored Research Award; Duke Department of Anesthesiology.
References
- 1. Yang R, Wolfson M, Lewis MC. Unique aspects of the elderly surgical population: an anesthesiologist's perspective. Geriatr Orthop Surg Rehabil. 2011;2(2):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327‐406. [DOI] [PubMed] [Google Scholar]
- 3. Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population‐based case‐control study. Alzheimers Dement. 2014;10(2):196‐204. [DOI] [PubMed] [Google Scholar]
- 4. Chen PL, Yang CW, Tseng YK, et al. Risk of dementia after anaesthesia and surgery. Br J Psychiatry. 2014;204(3):188‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avidan MS, Searleman AC, Storandt M, et al. Long‐term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fischer P, Wallner H, Jungwirth S, et al. Cumulative exposure to general anesthesias and cognitive dysfunction at age 75 in the Vienna Transdanube Aging "VITA" study. J Neuropsychiatry Clin Neurosci. 2007;19(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 7. Sprung J, Jankowski CJ, Roberts RO, et al. Anesthesia and incident dementia: a population‐based, nested, case‐control study. Mayo Clin Proc. 2013;88(6):552‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gasparini M, Vanacore N, Schiaffini C, et al. A case‐control study on Alzheimer's disease and exposure to anesthesia. Neurol Sci. 2002;23(1):11‐14. [DOI] [PubMed] [Google Scholar]
- 9. Harmanci H, Emre M, Gurvit H, et al. Risk factors for Alzheimer disease: a population‐based case‐control study in Istanbul, Turkey. Alzheimer Dis Assoc Disord. 2003;17(3):139‐145. [DOI] [PubMed] [Google Scholar]
- 10. Evered L, Silbert B, Scott DA, Ames D, Maruff P, Blennow K. Cerebrospinal fluid biomarker for Alzheimer disease predicts postoperative cognitive dysfunction. Anesthesiology. 2016;124(2):353‐361. [DOI] [PubMed] [Google Scholar]
- 11. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery‐2018. Br J Anaesth. 2018;121(5):1005‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newman MF, Kirchner JL, Phillips‐Bute B, et al. Longitudinal assessment of neurocognitive function after coronary‐artery bypass surgery. N Engl J Med. 2001;344(6):395‐402. [DOI] [PubMed] [Google Scholar]
- 13. Inouye SK, Marcantonio ER, Kosar CM, et al. The short‐term and long‐term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12(7):766‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Selnes OA, Grega MA, Bailey MM, et al. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63(5):581‐590. [DOI] [PubMed] [Google Scholar]
- 15. Selnes OA, Grega MA, Bailey MM, et al. Do management strategies for coronary artery disease influence 6‐year cognitive outcomes? Ann Thorac Surg. 2009;88(2):445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Browndyke JN, Berger M, Harshbarger TB, et al. Resting‐state functional connectivity and cognition after major cardiac surgery in older adults without preoperative cognitive impairment: preliminary findings. J Am Geriatr Soc. 2017;65(1):e6‐e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Browndyke JN, Berger M, Smith PJ, et al. Task‐related changes in degree centrality and local coherence of the posterior cingulate cortex after major cardiac surgery in older adults. Hum Brain Mapp. 2018;39(2):985‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26(4):231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sprung J, Kruthiventi SC, Warner DO, et al. Exposure to surgery under general anaesthesia and brain magnetic resonance imaging changes in older adults. Br J Anaesth. 2019;123(6):808‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kline RP, Pirraglia E, Cheng H, et al. Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology. 2012;116(3):603‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid‐beta oligomerization and cytotoxicity. Anesthesiology. 2004;101(3):703‐709. [DOI] [PubMed] [Google Scholar]
- 22. Xie Z, Culley DJ, Dong Y, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta‐protein level in vivo. Ann Neurol. 2008;64(6):618‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Freche H, Brouillette J, Fernandez‐Gomez FJ, et al. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012;116(4):779‐787. [DOI] [PubMed] [Google Scholar]
- 24. Planel E, Bretteville A, Liu L, et al. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J. 2009;23(8):2595‐2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whittington RA, Virág L, Gratuze M, et al. Dexmedetomidine increases tau phosphorylation under normothermic conditions in vivo and in vitro. Neurobiol Aging. 2015;36(8):2414‐2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whittington RA, Virág L, Marcouiller F, et al. Propofol directly increases tau phosphorylation. PLoS One. 2011;6(1):e16648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wan Y, Xu J, Meng F, et al. Cognitive decline following major surgery is associated with gliosis, beta‐amyloid accumulation, and tau phosphorylation in old mice. Crit Care Med. 2010;38(11):2190‐2198. [DOI] [PubMed] [Google Scholar]
- 28. Kamer AR, Galoyan SM, Haile M, et al. Meloxicam improves object recognition memory and modulates glial activation after splenectomy in mice. Eur J Anaesthesiol. 2012;29(7):332‐337. [DOI] [PubMed] [Google Scholar]
- 29. Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115(4):727‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berger M, Nadler JW, Friedman A, et al. The effect of propofol versus isoflurane anesthesia on human cerebrospinal fluid markers of Alzheimer's disease: results of a randomized trial. J Alzheimers Dis. 2016;52(4):1299‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palotas A, Reis HJ, Bogats G, et al. Coronary artery bypass surgery provokes Alzheimer's disease‐like changes in the cerebrospinal fluid. J Alzheimers Dis. 2010;21(4):1153‐1164. [DOI] [PubMed] [Google Scholar]
- 32. Reinsfelt B, Ricksten SE, Zetterberg H, Blennow K, Freden‐Lindqvist J, Westerlind A. Cerebrospinal fluid markers of brain injury, inflammation, and blood‐brain barrier dysfunction in cardiac surgery. Ann Thorac Surg. 2012;94(2):549‐555. [DOI] [PubMed] [Google Scholar]
- 33. Reinsfelt B, Westerlind A, Blennow K, Zetterberg H, Ricksten SE. Open‐heart surgery increases cerebrospinal fluid levels of Alzheimer‐associated amyloid beta. Acta Anaesthesiol Scand. 2013;57(1):82‐88. [DOI] [PubMed] [Google Scholar]
- 34. Ferreira D, Perestelo‐Perez L, Westman E, Wahlund LO, Sarria A, Serrano‐Aguilar P. Meta‐review of CSF core biomarkers in Alzheimer's disease: the state‐of‐the‐art after the new revised diagnostic criteria. Front Aging Neurosci. 2014;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta‐analysis. Lancet Neurol. 2016;15(7):673‐684. [DOI] [PubMed] [Google Scholar]
- 36. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence‐Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35(1):64‐101. [DOI] [PubMed] [Google Scholar]
- 37. Nobuhara CK, Bullock WM, Bunning T, et al. A protocol to reduce self‐reported pain scores and adverse events following lumbar punctures in older adults. J Neurol. 2020;267(7):2002‐2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaw LM, Waligorska T, Fields L, et al. Derivation of cutoffs for the Elecsys((R)) amyloid beta (1‐42) assay in Alzheimer's disease. Alzheimers Dement. 2018;10:698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuan Y. Multiple imputation using SAS software. J Stat Softw. 2011;45(6):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDonagh DL, Mathew JP, White WD, et al. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112(4):852‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279‐284. [DOI] [PubMed] [Google Scholar]
- 42. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaw LM, Vanderstichele H, Knapik‐Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang B, Tian M, Zheng H, et al. Effects of anesthetic isoflurane and desflurane on human cerebrospinal fluid Aβ and τ level. Anesthesiology. 2013;119(1):52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ballweg T, White M, Parker M, et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth. 2021;126(2):458‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Danielson M, Wiklund A, Granath F, et al. Association between cerebrospinal fluid biomarkers of neuronal injury or amyloidosis and cognitive decline after major surgery. Br J Anaesth. 2021;126(2):467‐476. [DOI] [PubMed] [Google Scholar]
- 49. Yaksh TL, Tozier N, Horais KA, et al. Toxicology profile of N‐methyl‐D‐aspartate antagonists delivered by intrathecal infusion in the canine model. Anesthesiology. 2008;108(5):938‐949. [DOI] [PubMed] [Google Scholar]
- 50. DeLeo JA, Colburn RW, Rickman AJ, Yeager MP. Intrathecal catheterization alone induces neuroimmune activation in the rat. Eur J Pain. 1997;1(2):115‐122. [DOI] [PubMed] [Google Scholar]
- 51. Olsson M, Ärlig J, Hedner J, Blennow K, Zetterberg H. Repeated lumbar punctures within 3 days may affect CSF biomarker levels. Fluids Barriers CNS. 2019;16(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ancelin ML, de Roquefeuil G, Ledesert B, Bonnel F, Cheminal JC, Ritchie K. Exposure to anaesthetic agents, cognitive functioning and depressive symptomatology in the elderly. Br J Psychiatry. 2001;178:360‐366. [DOI] [PubMed] [Google Scholar]
- 53. Berger M, Murdoch DM, Staats JS, et al. Flow cytometry characterization of cerebrospinal fluid monocytes in patients with postoperative cognitive dysfunction: a pilot study. Anesth Analg. 2019;129(5):e150‐e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP. Neurocognitive function after cardiac surgery: from phenotypes to mechanisms. Anesthesiology. 2018;129(4):829‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Devinney MJ, Mathew JP, Berger M. Postoperative delirium and postoperative cognitive dysfunction: two sides of the same coin? Anesthesiology. 2018;129(3):389‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Klinger RY, Cooter M, Bisanar T, et al. Intravenous lidocaine does not improve neurologic outcomes after cardiac surgery: a randomized controlled trial. Anesthesiology. 2019;130(6):958‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klinger RY, James OG, Borges‐Neto S, et al. 18F‐florbetapir positron emission tomography‐determined cerebral beta‐amyloid deposition and neurocognitive performance after cardiac surgery. Anesthesiology. 2018;128(4):728‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. VanDusen KW, Li YJ, Cai V, et al. Cerebrospinal fluid proteome changes in older non‐cardiac surgical patients with postoperative cognitive dysfunction. J Alzheimers Dis. 2021;80(3):1281‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mathew JP, Mackensen GB, Phillips‐Bute B, et al. Randomized, double‐blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40(3):880‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mathew JP, Mackensen GB, Phillips‐Bute B, et al. Effects of extreme hemodilution during cardiac surgery on cognitive function in the elderly. Anesthesiology. 2007;107(4):577‐584. [DOI] [PubMed] [Google Scholar]
- 62. Mathew JP, Podgoreanu MV, Grocott HP, et al. Genetic variants in P‐selectin and C‐reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49(19):1934‐1942. [DOI] [PubMed] [Google Scholar]
- 63. Mathew JP, White WD, Schinderle DB, et al. Intraoperative magnesium administration does not improve neurocognitive function after cardiac surgery. Stroke. 2013;44(12):3407‐3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Messerotti Benvenuti S, Zanatta P, Valfre C, Polesel E, Palomba D. Preliminary evidence for reduced preoperative cerebral blood flow velocity as a risk factor for cognitive decline three months after cardiac surgery: an extension study. Perfusion. 2012;27(6):486‐492. [DOI] [PubMed] [Google Scholar]
- 65. Moller JT, Cluitmans P, Rasmussen LS, et al. Long‐term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post‐Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857‐861. [DOI] [PubMed] [Google Scholar]
- 66. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18‐30. [DOI] [PubMed] [Google Scholar]
- 67. Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112(3):440‐451. [DOI] [PubMed] [Google Scholar]
- 68. Puskas F, Grocott HP, White WD, Mathew JP, Newman MF, Bar‐Yosef S. Intraoperative hyperglycemia and cognitive decline after CABG. Ann Thorac Surg. 2007;84(5):1467‐1473. [DOI] [PubMed] [Google Scholar]
- 69. Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(suppl 1):i98‐i105. [DOI] [PubMed] [Google Scholar]
- 70. Van Dijk D, Jansen EW, Hijman R, et al. Cognitive outcome after off‐pump and on‐pump coronary artery bypass graft surgery: a randomized trial. JAMA. 2002;287(11):1405‐1412. [DOI] [PubMed] [Google Scholar]
- 71. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 72. Kennedy RE, Cutter GR, Wang G, Schneider LS. Challenging assumptions about African American participation in Alzheimer disease trials. Am J Geriatr Psychiatry. 2017;25(10):1150‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mateen FJ. Is it time for quotas to achieve racial and ethnic representation in multiple sclerosis trials? Front Neurol. 2021;12:680912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Duff K. Evidence‐based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27(3):248‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rosner B. Fundamentals of Biostatistics. Thomson‐Brooks/Cole; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics of lost to follow‐up (LTFU) cohort compared to returning cohort at 6 weeks.
Table S2. Comparison of cognitive trajectories between entire cohort who returned for 6‐week follow‐up, and a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.
Table S3. Correlations between preoperative CSF biomarker levels and preoperative to 6‐week postoperative cognitive change, in the entire cohort who returned for 6‐week follow‐up, and in a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.
Table S4. Correlations between preoperative to 24‐h postoperative CSF biomarker change and preoperative to 6‐week postoperative cognitive change, in the entire cohort who returned for 6‐week follow‐up, and in a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.
Table S5. Correlations between preoperative to 6‐week postoperative CSF biomarker changes and preoperative to 6‐week postoperative cognitive change, in the entire cohort who returned for 6‐week follow‐up, and in a subset of n = 26 patients who returned for 6‐week follow‐up yet who had baseline characteristics similar to the patients lost to follow‐up prior to the 6‐week postoperative study visit.


