Abstract
Objective
Research is needed to determine best practice for genomic testing in the context of child interstitial or diffuse lung disease (chILD). We explored parent’s and child’s health-related quality of life (HRQoL), parents’ perceived understanding of a genomic testing study, satisfaction with information and the study and decisional regret to undertake genomic testing.
Methods
Parents of children with diagnosed or suspected chILD who were enrolled in a genomic sequencing study were invited to complete questionnaires pretesting (T1) and after receiving the result (T2).
Results
Parents’ (T1, n=19; T2, n=17) HRQoL was lower than population norms. Study satisfaction (T1) and perceived understanding (T2) were positively correlated (rs=0.68, p=0.014). Satisfaction with information (T1 and T2) and decisional regret (T2) were negatively correlated (T1 rs=−0.71, p=0.01; T2 rs=−0.56, p=0.03). Parents reported wanting more frequent communication with staff throughout the genomic sequencing study, and greater information about the confidentiality of test results.
Conclusions
Understanding of genomic testing, satisfaction with information and participation and decisional regret are inter-related. Pretest consultations are important and can allow researchers to explain confidentiality of data and the variable turnaround times for receiving a test result. Staff can also update parents when there will be delays to receiving a result.
Keywords: rare lung diseases, paediatric lung disaese
Key messages.
What is already known on this topic
Parental regret for undergoing genomic testing for their child’s illness is generally low and may be linked to poor understanding of testing.
What this study adds
This study shows that parents of children undergoing genomic testing for child interstitial or diffuse lung disease need more information about confidentiality and privacy of data, and updates throughout the process when there will be delays to receiving a result.
How this study might affect research, practice or policy
This research provides clinicians and genetic counsellors important information to support parents of children undergoing genomic testing for respiratory conditions.
Infant and child interstitial or diffuse lung disease (chILD) comprises a heterogeneous group of approximately 200 rare, chronic respiratory disorders characterised by abnormalities in the respiratory and immune systems.1 The estimated incidence of chILD is 0.13–16.2 cases per 100 000 children/year, although exact incidence rates are difficult to determine.2 chILD commonly presents as breathlessness, hypoxaemia and growth abnormalities in children.3 Morbidity can vary depending on the specific diagnosis and the severity of the respiratory compromise, but children with chILD may require care throughout their lifetime.2 3 The mortality rate in high-income countries has been found to range from 6% to 30% for young people under 18 years.2
chILD presents many challenges for patients and their parents. For instance, children with chILD may be regularly hospitalised, frequently absent from school, restricted in their functional or recreational activities and experience disrupted psychosocial development.4 5 Families may also experience uncertainty regarding treatments and prognosis.4 Previous research has shown that children with chILD have lower health-related quality of life (HRQoL) than their peers without chILD.5 Clinical factors related to poorer HRQoL among children with chILD include a higher Fan severity score, extrapulmonary involvement, need for long-term oxygen therapy, use of enteral nutritional support and higher number of oral therapies.5
The burden of disease and treatment may also impact parents’ HRQoL, however, research assessing the impact of chILD on families is lacking.2 Research in other illness groups has shown that parents’ poor mental health and well-being is associated with lower satisfaction with their child’s care, and lower understanding of the illness and treatments.6 7 As such, satisfaction with clinical care may therefore help buffer the impact of chILD on parents, although further research is needed to confirm this.
The aetiology of chILD is unknown in approximately 8%–27% of cases.8–11 Genomic testing may help clinicians and families to better understand the aetiology of chILD and may be used to recommend personalised treatment plans and avoid unnecessary biopsies.1 12 Nevertheless, managing parents’ expectations regarding genomic testing is important13 given that only 25%–50% of children will receive a diagnosis after testing for any condition.12 14 While regret for undergoing genomic testing is generally low, regret may be linked to parental confusion regarding the test and test result.14 This points to the importance of parental understanding of, and satisfaction with, the information provided to the family about genomic testing. Ensuring that parents are fully informed prior to consenting to genomic testing is therefore essential.15
Little is known about parents’ satisfaction with, and understanding of, genomic testing for children with chILD. By examining parents’ experiences with genomic testing for chILD, we can better determine areas for improvement to provision of emotional support and information during genetic consults.16 17
We aimed to explore:
HRQoL among parents and children enrolled in a genomic testing study for chILD, and whether parent’s and child’s HRQoL are related.
Parents’ (i) understanding of information provided to them about the genomic testing study, (ii) satisfaction with information provided to them about the genomic testing study, (iii) satisfaction with their participation in the study and (iv) their regret in deciding to participate in the study.
Factors related to parent’s and child’s HRQoL, and parent understanding, satisfaction and regret over the course of the genomic testing study.
Methods
This study was part of a larger evaluation of whole exome sequencing for children with chILD, titled chILDRANZ. The larger study aimed to understand the value of genomic sequencing for children with chILD and the impact on patient healthcare. The current study focuses on the psychosocial aspects of genomic sequencing from parents’ perspectives. Parents/Guardians (parents used hereafter) could opt-in to genomic sequencing for their child without having to complete the psychosocial questionnaires.
Participants
Children were eligible for the genomic test if they had either diagnosed or suspected interstitial or diffuse lung disease (chILD). Diagnostic labels are provided in online supplemental table 1. Suspected chILD included the following:
bmjresp-2021-001139supp001.pdf (69.2KB, pdf)
Respiratory symptoms (eg, cough, tachypnoea or dyspnoea) at rest or with exercise, crackles, retractions, clubbing, failure to thrive, respiratory failure, systemic arterial hypoxaemia.
Diffuse infiltrates on CT scanning.
Abnormal pulmonary function tests with evidence of restrictive ventilatory defect, and persistence of these findings for >4 weeks or when the condition is clinically suspected in the case of acute neonatal respiratory distress.18
Children were not eligible for the genomic test if they had cystic fibrosis, primary ciliary dyskinesia or other ‘common causes’ of diffuse lung disease. Children were also not eligible if they exhibited ‘masqueraders’ (eg, congenital heart failure).19
In addition to these inclusion and exclusion criteria, parents were eligible for the psychosocial study if they were able to speak and read conversational English.
Patient and public involvement
Patients were not involved in the development or design of this research.
Procedure
Potentially eligible children were identified by the child’s treating specialist during clinical care, through specialist clinical units or as inpatients at 19 Australian hospitals as part of the chILDRANZ network. The project manager provided the study genetic counsellor with the contact details of parents of eligible children. The study genetic counsellor then contacted the parents to discuss the study and consent procedure and to provide an information booklet and consent form. The information booklet provided to parents was 16 pages (including the consent form), and contained information about the purpose of the research, what would happen to their child’s data including DNA samples, what genomic testing is and the risks, benefits and potential outcomes of genomic testing (online supplemental file 2) (The information booklets at each site contained the same content, with site-specific logos and contact details; an example booklet from one site is provided in online supplemental file 2). The study genetic counsellor’s contact details were also provided on the information booklet in the event that a parent was distressed or if they wanted to discuss any of the information in the booklet. The project manager emailed parents a link to a baseline questionnaire within 1 month of consenting to the genomic test (T1) and a follow-up questionnaire 3–6 months after receiving the test result (T2). Parents completed the questionnaires online, which took approximately 20 min.
bmjresp-2021-001139supp002.pdf (349KB, pdf)
Measures
Parents completed a baseline questionnaire (T1) and a follow-up questionnaire (T2), which contained validated scales and purposively designed questions.
Sociodemographics
Parents recorded their sex, highest level of education, employment status, whether they have private health insurance and the age and sex of their child undergoing genomic testing.
Parents’ HRQoL
We used the 12-Item Short Form Survey version 2 (SF-12v2) to asses parents’ HRQoL.20 Respondents complete 12 items, with different rating scales. The SF-12v2 contains two subscales: the mental composite score (assessing mental functioning) and the physical composite score (assessing physical functioning). Scores are standardised,21 so that scores range from 1 to 100, with a mean of 50 and SD of 10. Higher scores indicate better mental and physical functioning. The SF-12v2 displays strong validity and test–rest reliability.20
Parent-reported HRQoL of the affected child
We used a measure of HRQoL designed specifically for children with chILD (chILD-QoL).4 We used the parent-proxy reports for the current study. The measure has six versions adapted according to the child’s age: 0–12 months (5 items), 13–24 months (8 items), 2–4 years (11 items), 5–7 years (11 items), 8–12 years (11 items), 13–18 years (11 items). Parents were asked how often over the previous month their child experienced a range of symptoms or issues (eg, ‘getting out of breath’), which parents rated on a scale from 1 (‘never’) to 5 (‘always’). Items were reverse-scored, linearly transformed to a scale of 0–100 and combined to form a total score including all age groups. Higher scores indicate higher HRQoL. The chILD-QoL has demonstrated strong internal reliability and convergent validity.4 In the current sample, Cronbach’s α ranged from 0.66 to 0.92 for the different versions.
Perceived understanding
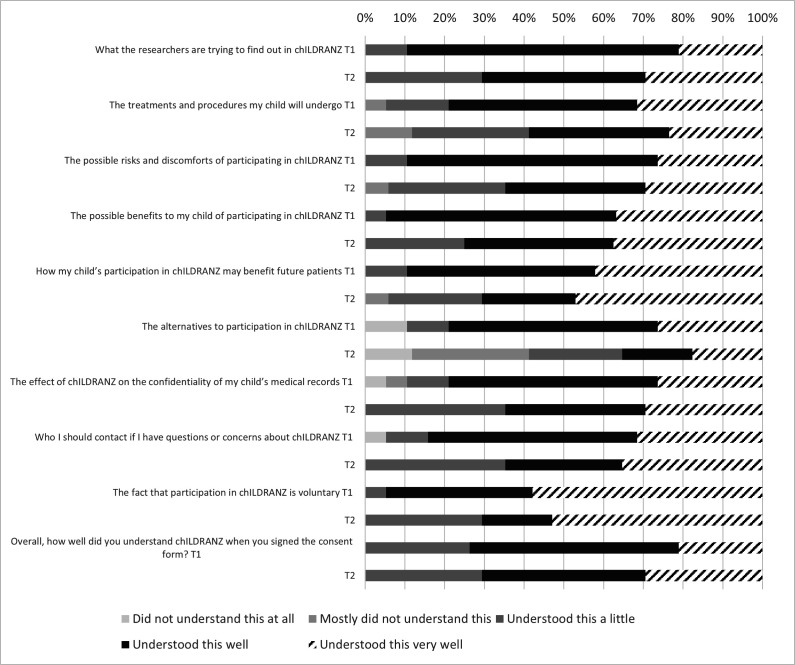
We assessed parents’ perceived understanding of the genomic sequencing study using purposively designed questions. Ten items (figure 1) asked parents to rate how well they understood different aspects of the chILDRANZ study on a scale from 1 (“I did not understand this at all”) to 5 (“I understood this very well”). Item responses were added together to create a total score (ranging from 10 to 50), with higher scores indicating higher parent-perceived understanding. The scale demonstrated strong internal reliability in the current study at both timepoints: T1 Cronbach’s α=0.85, T2 Cronbach’s α=0.94.
Figure 1.
Parents’ perceived understanding of key study elements.
Satisfaction with information
We assessed parents’ satisfaction with the information provided to them about the study using purposively designed questions. The question stem stated: ‘The information sheet and consent form was…’. Parents were then presented with six descriptors (clearly presented, informative, easy to read, useful to make a decision, visually appealing, easy to navigate) and asked to rate their agreement with each on a scale from 1 (‘very’) to 5 (‘not at all’). Responses were then reverse-scored and summed to create a total score. Higher scores indicated greater satisfaction with the information. Internal reliability was adequate-to-strong with the current sample: T1 Cronbach’s α=0.69; T2 Cronbach’s α=0.90.
Parents also completed open-ended questions and single items about their satisfaction with the information. Single items enquired whether parents were satisfied with the length of the information provided to them (1=too long; 2=just right; 3=too short), whether they needed help reading the information (1=yes, 2=no), if yes, who helped them read the information (open-ended) and how thoroughly they read the information (1=from cover to cover; 2=quite thoroughly; 3=just the parts I thought were relevant; 4=briefly; 5=I did not read the information). For this last question, we recoded the responses as 1=read thoroughly (answers 1–2) and 2=did not read thoroughly (answers 3–4) during analysis. Two scale items asked parents how worried and reassured the information made them feel, respectively, on a scale from 1 (‘not at all’) to 5 (‘very much’). Parents then completed two open-ended questions about what made them feel worried and/or reassured.
Satisfaction with participation
Parents also reported their overall satisfaction with their involvement in the study on a scale from 0 (“I had a very poor experience”) to 10 (“I had a very good experience”). One open-ended question enquired about areas for improvement.
Regret with decision to participate
We measured parents’ regret about participating in the genomic sequencing study with the Decision Regret Scale (DRS).22 The DRS consists of five statements of distress or remorse after making a healthcare decision (eg, “I would go for the same choice if I had to do it over again”). Parents rated how much they agreed with each statement on a 5-point Likert scale from 1 (‘strongly agree’) to 5 (‘strongly disagree’). Scores were then converted to a scale of 0 (no regret) to 100 (high regret).23 The DRS is validated, widely used and displays strong internal reliability and convergent validity.22 24 The DRS had strong internal reliability at both timepoints: T1 Cronbach’s α=0.92; T2 Cronbach’s α=0.72.
Data analysis
We used SPSS V.24.0 to analyse the data. We used descriptive statistics to assess parent’s sociodemographic status. We used Pearson’s correlation to assess the relationship between parent’s and child’s HRQoL, and Spearman’s Rho correlations to examine the relationships between understanding of the study, satisfaction with information, satisfaction with the study, decisional regret, parent’s HRQoL and child’s HRQoL. Mann-Whitney U tests were used to determine whether these factors differed according to parent level of education and how thoroughly they read the information.
We analysed parents’ responses to the open-ended questions using directed qualitative content analysis, whereby we used the qualitative data to extend and validate the quantitative data.25 We present illustrative quotes to supplement the quantitative findings.
Results
We sent the baseline survey (T1) to 34 parents whose children were enrolled in the larger genomic testing study, and 19 parents (58.8%) returned a completed questionnaire. After the genomic testing results were completed, we invited the 34 parents to complete a follow-up questionnaire (T2), and 17 parents (50.0%) returned a completed questionnaire. Fourteen parents completed the questionnaire at both T1 and T2.
At T1, 84% of parents were mothers (n=16). Affected children were mostly male (n=11, 58%) and aged 0–12 months (n=10, 53%). Sociodemographics are reported in table 1.
Table 1.
Sociodemographics of parent and their affected child
| Time 1 (n=19) | Time 2 (n=17) | |
| Parent sex | ||
| Female | 16 (84.2%) | 13 (76.5%) |
| Male | 3 (15.8%) | 3 (17.6%) |
| Prefer not to say | – | 1 (5.9%) |
| Parent highest level of education | ||
| High school | 5 (26.3%) | 1 (5.9%) |
| Beyond high school | 14 (73.7%) | 16 (94.1%) |
| Employment | ||
| Employed: full-time/part-time/casual | 10 (52.6%) | 10 (58.8%) |
| Not employed: actively seeking work/not seeking work/retired/student/home duties | 8 (42.1%) | 7 (41.2%) |
| Private health insurance | ||
| Yes | 9 (47.4%) | 9 (52.9%) |
| No (Medicare only) | 9 (47.4%) | 8 (47.1%) |
| Child age | ||
| 0–12 months | 10 (52.6%) | 7 (41.2%) |
| 13–24 months | 6 (31.6%) | 5 (29.4%) |
| 2–4 years | 2 (10.5%) | 4 (23.5%) |
| 5–7 years | 1 (5.3%) | 1 (5.9%) |
| Child sex | ||
| Female | 7 (36.8%) | 6 (35.3%) |
| Male | 11 (57.9%) | 11 (64.7%) |
Fourteen parents completed questionnaires at both timepoints.
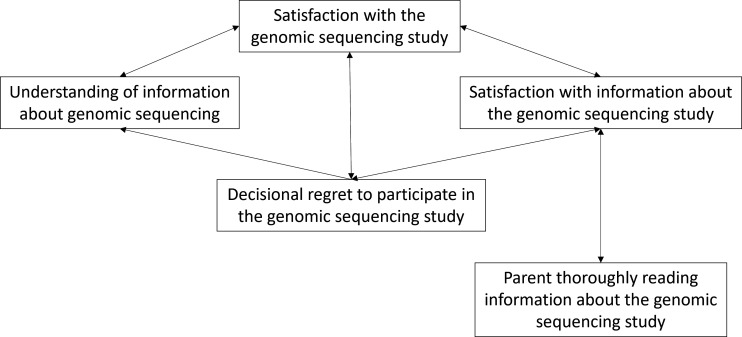
Parents report of satisfaction with the study, understanding of information provided to them, satisfaction with information and decisional regret were all inter-related as depicted in figure 2. Figure 2 outlines the correlations between these variables.
Figure 2.
Relationships between parent satisfaction, perceived understanding and decisional regret.
Parent’s and child’s HRQoL
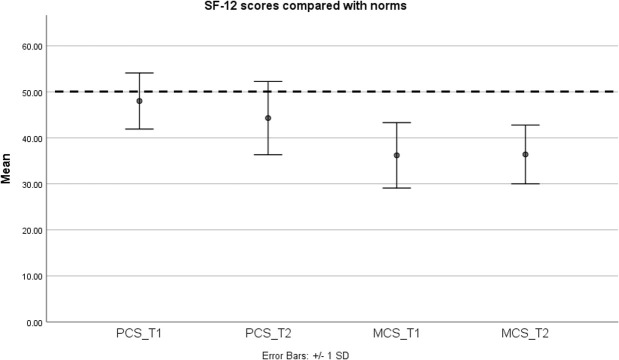
At T1 (M=36.50, SD=7.30) and T2 (M=37.44, SD=5.91), mean parent mental functioning scores as measured by the SF-12 were >1 SD lower than population norms (M=50 and SD=10; figure 3).
Figure 3.
Parents’ physical and mental functioning compared with norms. Dashed line, population mean; MCS_T1, mental composite score time 1; MCS_T2, mental composite score time 2; PCS_T1, physical composite score time 1; PCS_T2, physical composite score time 2; SF-12, 12-Item Short Form Survey.
Parent-reported child’s HRQoL was also low, with parents reporting M=38.45, SD=21.82 at T1, and M=44.08, SD=24.86 at T2.
Parent mental functioning at T1 was positively correlated with their report of their child’s HRQoL at T1 (r=0.62, p=0.01) and T2 (r=0.61, p=0.03). Parent’s and child’s HRQoL did not appear to be related to parents’ ratings of their understanding, satisfaction or decisional regret.
Understanding of the study
Parents reported overall high understanding of the study, with a median total score of 40/50 (IQR=39, 46) at T1 and 41/50 at T2 (IQR=30.5, 44.75).
Figure 1 shows how well parents reported understanding each aspect of the study. The percentage of parents reporting that they understood each aspect of the study ‘well’ or ‘very well’ appeared to decrease from T1 to T2. Parents particularly reported not understanding the alternatives to chILDRANZ at T2, with 79% at T1 and 41% at T2 reporting understanding this ‘well’/‘very well’.
At T1, parents with a high school level education reported higher perceived understanding of the information about the study than parents who were educated beyond high school: Mann-Whitney U test=11.50, SE=9.94, p=0.035.
Satisfaction with information
Parents were generally satisfied with the information provided to them about the study, with a median total score of 26/30 for both T1 (IQR=25, 29) and T2 (IQR=23, 29). At T1, most parents felt the length of the information was ‘just right’ (n=12, 63%) while 6 (32%) felt it was too long. Just one parent reported they needed help to read the information from a genetic counsellor. Eleven parents (58%) reported thoroughly reading the information provided about the study, while eight parents (42%) briefly read the information.
Parents who read the information thoroughly at T1 reported greater satisfaction with the information they received than parents who briefly read the information: Mann-Whitney U test=18.00, SE=11.92, p=0.033.
Most parents reported the information helped them feel at least ‘a little’ reassured (T1=17, 90%; T2=13, 77%). Parent comments showed they felt reassured because they were able to ask professionals questions (“The fact that we had a genetic counsellor to speak it over with”), they could receive an explanation for their child’s illness (“The possibility of finding a diagnosis”) and could help other families (“That we were making the right decision for our family and to hopefully help future families”).
Several parents revealed that the information made them feel at least ‘a little’ worried (T1=8, 42%; T2=7, 41%). Parents’ comments revealed that they were worried about the test result (“The thought that my child may have a lifelong condition”). Parents were also worried that the test results may impact their child in the future (“May affect insurance later in life”) and about the privacy of their child’s genetic information.
Making sure that information about my son was only used in this research, I would hate to think at some later point having his genetic information on ‘file’ may have a negative impact on some aspect of his life.
Satisfaction with participation
Parents were generally satisfied with their participation, with parents reporting a median score of 8/10 at both T1 (IQR=7, 10) and T2 (IQR=6, 10). Higher satisfaction was related to lower decisional regret at T1 (rs=−0.48, p=0.038).
Eight parents (42%) at T1 and five parents (29%) at T2 reported no regret at all regarding their participation in the genomic sequencing study. Levels of regret in the remaining parents were low with a median score of 12.5/100 at T1 (IQR=0, 25) and 10/100 at T2 (IQR=0, 23.75).
Parents’ higher perceived understanding of the study at T2 was correlated with higher satisfaction with the study at T1 (rs=0.68, p=0.014), and lower decisional regret at T1 (rs=−0.60, p=0.038). Higher decisional regret at T2 was correlated with lower satisfaction with information at T1 (rs=−0.71, p=0.01) and T2 (rs=−0.56, p=0.03).
Parents’ comments regarding dissatisfaction with the study revealed that lack of communication was the most frequent complaint. Parents wanted to be kept updated about delays to receiving the test result.
There was a disconnect in communication from when we were discharged [from the hospital] to when we started the trial…The genetics team were extremely hard to get a hold of or communicate with. From all the teams we dealt with they were the most difficult and stressful in regards to receiving information about the process of this trial…On numerous occasions the genetics team said they would come back to the ward to update us on [my daughter’s] next steps, however they never did.
The time it takes to get results or be updated on where the results are at. This took a lot of follow up.
Discussion and conclusion
Discussion
In this study, we aimed to explore (1) the HRQoL of parents and children enrolled in a genomic sequencing study for chILD, (2) parents’ understanding, satisfaction and decisional regret regarding their participation in the genomic testing study and (3) factors related to HRQoL, parent understanding, satisfaction and decisional regret.
Parents reported a mean mental functioning score >1 SD below population norms. Research on the mental health of parents of children with chILD is limited,2 so the current study adds to our understanding of the potential psychosocial toll of chILD on parents. Parent-reported child’s HRQoL was also lower than what has been reported in previous literature.4 26 Parents in the current study may have been more likely to complete the psychosocial questionnaire if their child was particularly unwell. Consistent with previous findings from other illness groups, parent mental functioning was lower when their perception of their child’s HRQoL was also low.27
In general, parents’ perceived understanding of, and satisfaction with, the study was high, and decisional regret was low. Parents’ satisfaction with having participated in the genomic testing study was related to having a greater understanding of the study, higher satisfaction with the information provided about the study and lower decisional regret about taking part in the study. A unique finding from this study was how study satisfaction, understanding, satisfaction with information and decisional regret were all inter-related (figure 2). Previous genomic testing studies have found that simple, clear information about testing can facilitate understanding and information retention.28 29
Parents reported that the alternatives to participating in the study was the least understood aspect of the information provided to them. Confidentiality of medical records was also quantitatively one of the least understood aspects of the study, according to parents. Qualitatively, parents reported being worried about the privacy of their child’s genetic information, and the future insurance implications of their child’s test result, which has been found among other clinical populations.29 30 These findings suggest that parents require further information about what would happen to their child’s care if they chose not to have genomic testing, and confidentiality and privacy of test results. This information could be discussed with genetic counsellors, and also provided in writing so that parents can refer back to this information at later points.29
We found that, at baseline, parents with a high school level education reported greater perceived understanding of the study than parents who were educated beyond high school. Parents’ median self-rated understanding of the study was generally quite high (40/50). As such, lower scores on ‘understanding’ may not reflect a lack of understanding of the study, but perhaps that they had more unanswered questions. Previous research has shown that patients with higher health literacy ask more questions in medical consultations, and are more likely to seek out further information than patients with low literacy.31 32 Considering that higher health literacy is related to higher formal education,33 this could explain our finding.
Parents’ open-ended responses revealed that communication between families and clinicians may be an important contributor to parent satisfaction with genomic sequencing. Previous research has also found that parents are less satisfied with clinical services related to their child’s genetic testing when the parent-clinician communication is inconsistent and sporadic.34 Parents in our study especially wanted delays in receiving the result of their child’s genomic test to be communicated to them, which has been found previously.35
Practice implications
Our findings have key implications for clinical practice and research regarding communication between clinicians and parents of children with chILD undergoing genomic testing. First, genetic counsellors (or in the case of a research project, the study team) may ensure the parents understand how and where their child’s results will be stored, who can access these data and whether the results will affect their child’s insurance during the pretest consultation. Genetic counsellors may discuss with parents the variable turnaround times to receiving a result of a genomic test during the pretest consultation.35 Genetic counsellors or the study team may also encourage parents to fully read the information provided to them prior to making a decision. The use of visual aids in these information booklets may be an important way to make the information more digestible.28 29 Genetic counsellors or the study team may also briefly assess parents’ satisfaction with information after initial genetic consultation. Parents with low satisfaction may require further information and consultation.
Communication between parents and genetic counsellors or the study team while waiting for the test results could also be an important way to help parents feel satisfied with their decision to participate in genomic testing. Genetic counsellors or the study team could consider keeping parents updated about delays to help manage their expectations and associated distress.
Limitations
The small sample size means further research is needed in order to confirm our findings. Research is particularly needed to assess the generalisability of our findings among families undergoing genetic evaluation in a clinical setting. The small sample precluded us from statistically assessing whether parents’ understanding, satisfaction and HRQoL changed over the course of the study, or whether these factors are related to the child’s test result.14 Larger longitudinal research is needed given that a greater percentage of participants appeared to report understanding aspects of the study ‘well’ or ‘very well’ at T1 than T2 (figure 1). Nevertheless, chILD are rare conditions, and as such any data on this topic are valuable. Another limitation of the study was that we used a subjective measure of parent understanding. An objective measure would have helped elucidate further how well parents understood important aspects of the study. We also did not assess parent understanding of the test result, which may have shed further light on parent understanding of genomic testing for chILD. Our sample may have been biased, with 59% of parents of children undergoing genomic testing choosing to complete the questionnaire. Similarly, our sample was limited to English-speaking parents and fathers were under-represented. Finally, including a comparison group of caregivers of children with other chronic childhood conditions would have helped to contextualise our findings.
Conclusion
The current study provides novel insights regarding families undergoing genomic testing for diagnosed or suspected chILD. We found that perceived understanding of the genomic testing study, satisfaction with the information they received, satisfaction with participation and decisional regret were inter-related. Clear, concise information about genomic testing—including what happens to genomic data, possible wait times and implications for insurance— may help parents to feel more satisfied with their decision to enrol their child in a genomic testing study. Information about privacy and confidentiality could also be discussed during pretest consultation with genetic counsellors. Genetic counsellors or the study team could also keep parents updated when there will be delays to receiving a result from the genomic test.
Acknowledgments
We would like to thank all the physicians, genetic counsellors, laboratory personnel and patients who took part in the children with interstitial lung disease research in Australia and New Zealand (chILDRANZ). We would like to thank Gadiel Dumlao for his contribution to the manuscript. We would like to thank chILD-EU for allowing us to use their chILD-QoL measure. We would like to acknowledge Luminesce Alliance—Innovation for Children’s Health for its contribution and support. Luminesce Alliance—Innovation for Children’s Health, is a not for profit cooperative joint venture between the Sydney Children’s Hospitals Network, the Children’s Medical Research Institute and the Children’s Cancer Institute. It has been established with the support of the NSW Government to coordinate and integrate paediatric research. Luminesce Alliance is also affiliated with the University of Sydney and the University of New South Wales Sydney. The Chair in Genomic Medicine awarded to JC is generously supported by The Royal Children’s Hospital Foundation. The research conducted at the Murdoch Children’s Research Institute was supported by the Victorian Government’s Operational Infrastructure Support Programme.
Footnotes
Contributors: LK: writing—original draft, formal analysis. CW: writing—review and editing, supervision. NV: project administration, writing—review and editing, investigation. DSA: methodology, investigation, resources. BB: writing—review and editing, investigation, resources. KB: writing—review and editing, investigation, resources. JC: writing—review and editing, investigation, resources. JH: writing—review and editing, investigation, resources. GH: methodology, investigation, resources. NK: writing—review and editing, investigation, resources. SL-T: investigation, resources. TMcD: methodology, investigation, resources. DM: writing—review and editing, methodology, investigation, resources. AS: writing—review and editing, investigation, resources. HS: writing—review and editing, investigation, resources. AT: writing—review and editing, investigation, resources. AJ: guarantor, conceptualisation, methodology, writing—review and editing, supervision, funding acquisition.
Funding: This study was funded by Australian Genomics HealthCare Alliance. Australian Genomics is funded by the NHMRC (GNT1113531, GNT2000001) and the Australian Government’s Medical Research Future Fund. CW is supported by the National Health and Medical Research Council of Australia (APP1143767). LK is supported by the Golda Meir Post-Doctoral Fellowship Fund.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Deidentified participant data are available from the corresponding author (https://orcid.org/0000-0001-9428-8807) on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
Ethics approval
This study was approved by Melbourne Health Human Research Ethics Committee (HREC: 36378). Participants gave informed consent to participate in the study before taking part.
References
- 1.Cunningham S, Jaffe A, Young LR. Children's interstitial and diffuse lung disease. Lancet Child Adolesc Health 2019;3:568–77. 10.1016/S2352-4642(19)30117-8 [DOI] [PubMed] [Google Scholar]
- 2.Hime NJ, Zurynski Y, Fitzgerald D, et al. Childhood interstitial lung disease: a systematic review. Pediatr Pulmonol 2015;50:1383–92. 10.1002/ppul.23183 [DOI] [PubMed] [Google Scholar]
- 3.Clement A, de Blic J, Epaud R, et al. Management of children with interstitial lung diseases: the difficult issue of acute exacerbations. Eur Respir J 2016;48: :1559–63. 10.1183/13993003.01900-2016 [DOI] [PubMed] [Google Scholar]
- 4.Niemitz M, Schwerk N, Goldbeck L, et al. Development and validation of a health-related quality of life questionnaire for pediatric patients with interstitial lung disease. Pediatr Pulmonol 2018;53:954–63. 10.1002/ppul.24018 [DOI] [PubMed] [Google Scholar]
- 5.Lauby C, Boelle P-Y, Abou Taam R, et al. Health-Related quality of life in infants and children with interstitial lung disease. Pediatr Pulmonol 2019;54:828–36. 10.1002/ppul.24308 [DOI] [PubMed] [Google Scholar]
- 6.Kelada L, Wakefield CE, Muppavaram N, et al. Psychological outcomes, coping and illness perceptions among parents of children with neurological disorders. Psychol Health 2021;36:1480–96. 10.1080/08870446.2020.1859113 [DOI] [PubMed] [Google Scholar]
- 7.Cartland J. Understanding the role of hospital design on the psychological trauma of hospitalization for children. Chicago: Luri Children’s Hosptial of Chicago Research Center, 2013. [Google Scholar]
- 8.Nathan N, Taam R, Epaud R, et al. A national internet-linked based database for pediatric interstitial lung diseases: the French network. Orphanet J Rare Dis 2012;7:40–11. 10.1186/1750-1172-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griese M, Seidl E, Hengst M, et al. International management platform for children's interstitial lung disease (chILD-EU). Thorax 2018;73:231–9. 10.1136/thoraxjnl-2017-210519 [DOI] [PubMed] [Google Scholar]
- 10.Fan LL, Dishop MK, Galambos C, et al. Diffuse lung disease in biopsied children 2 to 18 years of age. Application of the chILD classification scheme. Ann Am Thorac Soc 2015;12:1498–505. 10.1513/AnnalsATS.201501-064OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saddi V, Beggs S, Bennetts B, et al. Childhood interstitial lung diseases in immunocompetent children in Australia and New Zealand: a decade’s experience. Orphanet J Rare Dis 2017;12:1–9. 10.1186/s13023-017-0637-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark Z, Lunke S, Brett GR, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med 2018;20:1554–63. 10.1038/gim.2018.37 [DOI] [PubMed] [Google Scholar]
- 13.Donohue KE, Dolan SM, Watnick D, et al. Hope versus reality: parent expectations of genomic testing. Patient Educ Couns 2021;104:2073–9. 10.1016/j.pec.2021.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cakici JA, Dimmock DP, Caylor SA, et al. A prospective study of parental perceptions of rapid whole-genome and -Exome sequencing among seriously ill infants. Am J Hum Genet 2020;107:953–62. 10.1016/j.ajhg.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levenseller BL, Soucier DJ, Miller VA, et al. Stakeholders' opinions on the implementation of pediatric whole exome sequencing: implications for informed consent. J Genet Couns 2014;23:552–65. 10.1007/s10897-013-9626-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartley N, Best M, Jacobs C, et al. Cancer patients' views and understanding of genome sequencing: a qualitative study. J Med Genet 2020;57:671–6. 10.1136/jmedgenet-2019-106410 [DOI] [PubMed] [Google Scholar]
- 17.Lim Q, McGill BC, Quinn VF, et al. Parents' attitudes toward genetic testing of children for health conditions: a systematic review. Clin Genet 2017;92:569–78. 10.1111/cge.12989 [DOI] [PubMed] [Google Scholar]
- 18.Bush A, Cunningham S, de Blic J, et al. European protocols for the diagnosis and initial treatment of interstitial lung disease in children. Thorax 2015;70:1078–84. 10.1136/thoraxjnl-2015-207349 [DOI] [PubMed] [Google Scholar]
- 19.Kurland G, Deterding RR, Hagood JS, et al. An official American thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med 2013;188:376–94. 10.1164/rccm.201305-0923ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware J, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 21.Ware ME, Kosinski M, Dewey JE. How to score version 2 of the SF-36 health survey (standard & acute forms. Quality Metric Incorporated, 2001. [Google Scholar]
- 22.Brehaut JC, O'Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making 2003;23:281–92. 10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- 23.O’Connor A. User manual-decision regret scale. Ottawa: Ottawa Hospital Research Institute, 1996. [Google Scholar]
- 24.Becerra Pérez MM, Menear M, Brehaut JC, et al. Extent and predictors of decision regret about health care decisions: a systematic review. Med Decis Making 2016;36:777–90. 10.1177/0272989X16636113 [DOI] [PubMed] [Google Scholar]
- 25.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 26.Niemitz M, Schrader M, Carlens J, et al. Patient education for children with interstitial lung diseases and their caregivers: a pilot study. Patient Educ Couns 2019;102:1131–9. 10.1016/j.pec.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 27.Klassen AF, Klaassen R, Dix D, et al. Impact of caring for a child with cancer on parents' health-related quality of life. J Clin Oncol 2008;26:5884–9. 10.1200/JCO.2007.15.2835 [DOI] [PubMed] [Google Scholar]
- 28.Veilleux S, Bouffard M, Bourque Bouliane M. Patient and health care provider needs and preferences in understanding pharmacogenomic and genomic testing: a Meta-Data analysis. Qual Health Res 2020;30:43–59. 10.1177/1049732319858325 [DOI] [PubMed] [Google Scholar]
- 29.Lewis C, Sanderson S, Hill M, et al. Parents' motivations, concerns and understanding of genome sequencing: a qualitative interview study. Eur J Hum Genet 2020;28:874–84. 10.1038/s41431-020-0575-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amendola LM, Robinson JO, Hart R, et al. Why patients decline genomic sequencing studies: experiences from the CSER Consortium. J Genet Couns 2018;27:1220–7. 10.1007/s10897-018-0243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz MG, Jacobson TA, Veledar E, et al. Patient literacy and question-asking behavior during the medical encounter: a mixed-methods analysis. J Gen Intern Med 2007;22:782–6. 10.1007/s11606-007-0184-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menendez ME, van Hoorn BT, Mackert M, et al. Patients with limited health literacy ask fewer questions during office visits with hand surgeons. Clin Orthop Relat Res 2017;475:1291–7. 10.1007/s11999-016-5140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams RJ, Appleton SL, Hill CL, et al. Risks associated with low functional health literacy in an Australian population. Med J Aust 2009;191:530–4. 10.5694/j.1326-5377.2009.tb03304.x [DOI] [PubMed] [Google Scholar]
- 34.Nevin SM, Wakefield CE, Barlow‐Stewart K, et al. Psychosocial impact of genetic testing on parents of children with developmental and epileptic encephalopathy. Dev Med Child Neurol 2022;64:95–104. 10.1111/dmcn.14971 [DOI] [Google Scholar]
- 35.Nevin SM, McLoone J, Wakefield CE, et al. Genetic Testing in the Pediatric Nephrology Clinic: Understanding Families’ Experiences. J Pediatr Genet 2020;28. 10.1055/s-0040-1721439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2021-001139supp001.pdf (69.2KB, pdf)
bmjresp-2021-001139supp002.pdf (349KB, pdf)
Data Availability Statement
Data are available on reasonable request. Deidentified participant data are available from the corresponding author (https://orcid.org/0000-0001-9428-8807) on reasonable request.