Abstract
The role of lipids in neuroglial function is gaining momentum in part due to a better understanding of how many lipid species contribute to key cellular signalling pathways at the membrane level. The description of lipid rafts as membrane domains composed by defined classes of lipids such as cholesterol and sphingolipids has greatly helped in our understanding of how cellular signalling can be regulated and compartmentalized in neurons and glial cells. Genetic conditions affecting the metabolism of these lipids greatly impact on how some of these signalling pathways work, providing a context to understand the biological function of the lipid. Expectedly, abnormal metabolism of several lipids such as cholesterol and galactosyl-sphingolipids observed in several metabolic conditions involving lysosomal dysfunction are often accompanied by neuronal and myelin dysfunction. This review will discuss the role of lysosomal biology in the context of deficiencies in the metabolism of cholesterol and galactosyl-sphingolipids and their impact on neural function in three genetic disorders: Niemann-Pick type C, Metachromatic leukodystrophy and Krabbe’s disease.
Keywords: Cholesterol, Psychosine, Sulfatides, Lipids, Metabolism, Lysosomes, Myelin, Lipid raft, Endosome, Exosome, Signalling, Akt, Niemann-Pick type C disease, Krabbe’s disease, Metachromatic leukodystrophy
1. Introduction
Inborn errors of the metabolism of lipids are generally accompanied by lysosomal storage of undegraded material, affecting the physiology of multiple organs and systems (Parenti et al., 2013). Most often, patients develop manifestations early in life, leading to severe neurological and lethal infantile forms. Late onset variants of these diseases may also develop later in life and are often associated with cognitive and behavioural problems (Kolodny and Cable, 1982). Some of adult onset lipidoses have manifestations also present in other neurodegenerative diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS) suggesting that the involvement of common cellular pathogenic mechanisms (Kiriyama and Nochi, 2015; Ramanan and Saykin, 2013). The hallmarks of lysosomal storage diseases (LSD) include lysosomal dysfunction due to storage of specific lipids, dysmyelination and/or demyelination and neurodegeneration (Table 1). Due to their involvement in myelin production and lipid requirements, oligodendrocytes and Schwann cells are chiefly targeted in many lipidoses, which is also accompanied by neuronal and axonal abnormalities. In this review, due to the wide range of human diseases with errors of lipid metabolism, we will focus on some of the altered signalling and pathways that have been descried in three pathologies: Nieman-Pick type C (NPC), Metachromatic leukodystrophy (MLD) and Krabbe’s disease (KD).
Table 1.
Examples of Lysosomal storage diseases.
| Enzyme | Disease | Inheritance | Symptoms | Affected lipid |
|---|---|---|---|---|
| Galactosylceramidase | Krabbe’s disease | Autosomal recessive | Muscle weakness, stiff limbs, trouble walking, vision and hearing loss, muscle spasms, seizures. | Psychosine |
| Arylsulfatase A | Metachromatic leukodystrophy | Autosomal recessive | Loss of feeling in hands and feet, seizures, trouble walking and talking, vision and hearing loss. | Sulfatides |
| Acid sphingomyelinase (Type A, B); NPC1 and 2 (Type C1 and C2 respectively) | Niemann-Pick disease (A–C) | Autosomal recessive | hepatosplenomegaly, jaundice, slow development, trouble moving eyes up and down, breathing problems, heart disease. | Cholesterol and glycolipids |
| Glucocerebrosidase | Gaucher’s Disease | Autosomal recessive | Anemia, hepatosplenomegaly, easy bleeding and bruising, tiredness, bone and joint pain, seizures. | Glucocerebroside |
| α-Galactosidase A | Fabry’s Disease | X-linked | Pain, numbness, tingling n hands and feet, ringing in the ears, trouble breathing, dizziness, abnormal heart rhythms, stroke. | Glycosphingolipids |
| α-L-iduronidase (I); Iduronate sulfatase (II); N-acetyl galactosamine-4 sulfatase (VI); Heparan sulfamidase (III A); N-acetylglucosaminidase (III B); β-galactosidase (IVB); β-glucuronidase (VII); Hyaluronidase (IX) | Mucopoly-saccharidosis (MPS I-IX) | Most Autosomal recessive except MPS II (Hunter Syndrome) which is X-linked. | Intellectual disability, learning difficulties (I, II), Skeletal dysplasia (IV, VI, VII), motor dysfunction (III, IV, VI), trouble hearing and speaking, heart issues, trouble breathing and depression. | Heparan sulfate (I, II, III, VII), Dermatan sulfate (I, II, III, VI, VII), Keratan sulfate (IV, V) and Chondrotin sulfate (IV, VII). |
| α-Glucosidase | Pompe’s disease | Autosomal recessive | Poor muscle tone, severe muscle weakness, enlarged heart, liver, tongue. | Glycogen |
| Hexosaminidase A | Tay Sachs’ disease | Autosomal recessive | Progressive cognitive and motor deterioration resulting in seizures, intellectual disability, paralysis and death by age 5 years. Vision and hearing loss, red spot in the back of the eye are also common. | GM2 Ganglioside |
| Hexosaminidase A and B | Sandhoff’s disease | Autosomal recessive | Progressive cerebral degeneration beginning at 6 months, accompanied by blindness, red macular spot, hyperacusis. Almost indistinguishable from Tay Sachs except visceral involvement. | GM2 Ganglioside |
| Lysosomal acid lipase | Wolman’s disease | Autosomal recessive | Manifests within first few weeks of life with poor feeding, vomiting, abdominal distention secondary to hepatosplenomegaly; calcification of adrenal gland | Cholesteryl esters and triglycerides |
2. Generalities on lysosomal biology. Understanding possible secondary targets affected in LSD
While this review focuses on dysregulation of lysosomal lipid metabolism in NPC, MLD and KD, several key aspects of lysosome function and regulation including lysosome transport, signalling events at its surface and interaction with other organelles, are affected by lipid homeostasis (Settembre and Ballabio, 2014; Thelen and Zoncu, 2017). For this reason, we provide the readers with an introductory description of lysosome structure, function, its transcriptional regulation, key signalling processes that occur on the lysosomal surface and its interaction with other organelles. In each case, we also emphasize its relevance to lipid metabolism. Lastly, we briefly highlight other neurodegenerative diseases such as Parkinson’s disease (PD) that include lysosome dysfunction as part of their pathology, as this may bear relevance to the diseases discussed here as well. Indeed, in many of these neurodegenerative diseases, dysregulation in lysosomal lipid metabolism is a critical part of their pathology.
2.1. Lysosomes structure and function
Lysosomes, meaning “digestive body” in Greek, were discovered by the biochemist Christian De Duve in 1955 (de Duve, 2005; De Duve et al., 1955). Due to their role in terminal degradation, they are often thought of as the cell’s “garbage disposal system” and also thought to be relatively stable compartments. Lysosomes participate in numerous cellular processes including gene regulation, metabolic signalling, plasma membrane repair, immunity, cell adhesion and migration (Ballabio and Bonifacino, 2020; Saftig and Klumperman, 2009). They are also highly dynamic organelles whose number, shape, size and position can vary depending on external and internal cues (Ballabio and Bonifacino, 2020).
De Duve and colleagues first isolated the organelle from liver cells by the sucrose gradient centrifugation method. They worked out a five-fraction centrifugation process, refining on the process previously developed by Albert Claude at Rockefeller University, to isolate the “L” fraction enriched for acid phosphatase activity (De Duve et al., 1955). By 1955, they had discovered five acid hydrolases in this fraction, including the acid phosphatase; β-glucuronidase, Cathepsin D, ribonuclease and deoxyribonuclease. The presence of multiple hydrolases targeting very different substrates within these membrane-enclosed ‘sacs’ strongly implied that these were digestive organelles- hence named ‘Lysosome’ by De Duve and colleagues. Thus, originally identified by biochemists, lysosomes were then characterized morphologically, by electron microscopy studies of purified preparations from liver cells by Novikoff and colleagues (Novikoff et al., 1956). Novikoff subsequently developed a cytochemical staining method for acid phosphatase as a marker of lysosomes (Novikoff et al., 1956).
Historically, lysosome dysfunction has been associated only with rare LSDs that result from genetic impairment in of different lysosomal hydrolases (Ballabio and Gieselmann, 2009; Platt et al., 2018) (see Table 1). However, in recent years, it has become apparent that lysosome dysfunction is associated with numerous neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Amyotrophic Lateral Sclerosis (ALS) as well as several metabolic disorders (Malik et al., 2019; Nixon, 2020). This has led to lysosome biology becoming a central topic in fields beyond cell biology, such as neuroscience as well as physiology.
2.2. Lysosome composition
Lysosomes are composed of specific luminal, integral membrane proteins as well as peripherally associated proteins (Fig 1). Lysosomes have over 40 different membrane proteins (Callahan et al., 2009; Xu and Ren, 2015). LAMP (Lysosome associated membrane protein) 1 and 2 are two highly glycosylated proteins that are the most abundant lysosomal membrane proteins followed by lysosomal integral membrane protein 2 (LIMP; also called SCARB2) and the tetraspanin, CD63. LAMP1 and 2 form a glycocalyx that protects the lysosomes from its own very acidic environment (pH 4.5–5) and the activity of numerous catabolic enzymes (Eskelinen et al., 2004). These integral membrane proteins participate in many lysosomal functions, including membrane fusion events, motor-based transport of lysosomes and lysosome acidification (Ballabio and Bonifacino, 2020; Saftig and Klumperman, 2009). These membrane proteins also aid in cholesterol, ions and amino acid transport, protein import from cytosol, transport of degradation products to cytosol, and nutrient sensing (through interactions with peripherally associated proteins) (Amick et al., 2020). The soluble lysosomal proteins include more than 60 different enzymes (soluble hydrolases) that target specific substrates for degradation (Lubke et al., 2009). In addition to degrading their substrates, these enzymes are also involved in degradation of extracellular matrix (upon lysosome exocytosis), initiating apoptosis as well as antigen presentation (Conus and Simon, 2008). NPC2 is also a soluble lysosomal protein shuttles free cholesterol to NPC1, the integral membrane protein key for exporting this lipid from lysosomes (Hamer et al., 2012; Li and Pfeffer, 2016). These proteins and their role in cholesterol transport are discussed in detail later in this review.
Fig. 1.
Lysosomal components and properties. Lysosomes are composed of soluble proteins (includes numerous enzymes such as cathepsins), membrane proteins as well as peripherally associated proteins (on its cytosolic surface). Lysosomes have a highly acidic pH that is essential for the action of many of its enzymes. The pH is maintained by the activity of the vacuolar proton pump. Lysosomal membrane proteins include the highly glycosylated LAMP1 and LAMP2 proteins that form a glycocalyx lining the organelle. Other membrane proteins include the cholesterol and amino acid as well as ion transporters. Peripherally associated proteins include small GTPases such as Rab7 (involved in its movement, tethering to other organelles as well as fusion with other membranes).
Soluble and integral lysosomal proteins are delivered to the lysosome via the specific routes of vesicular transport from the Golgi (Pols et al., 2013; Swetha et al., 2011). The detailed mechanisms of transport and delivery of lysosomal proteins is reviewed elsewhere (Ballabio and Bonifacino, 2020; Saftig and Klumperman, 2009). Other proteins and protein complexes, including transcription factors, mTORC1 complex, adaptors for motor-base transport, small GTPases, etc. dynamically associate with the cytosolic side of the lysosome surface and help execute the relevant roles (Ballabio and Bonifacino, 2020; Saftig and Klumperman, 2009).
Like with proteins, lipids are an integral part of lysosomal membranes and affect the organelle’s function (Hamer et al., 2012). Reciprocally, lysosomes play an important role in cellular lipid homeostasis. Both endogenous and exogenous lipids get delivered to lysosomes. Exogenous lipids include lipoproteins and cholesteryl esters derived from LDLs (low density lipoproteins). These are processed by lysosomal acid lipase (Jaishy and Abel, 2016). Endogenous lipids such as from lipid droplets are delivered to lysosomes through the autophagic pathway and also processed by the lysosomal acid lipase that acts at an acidic pH (Settembre and Ballabio, 2014). Deficiency of this enzyme leads to Wolman disease, a lysosomal storage disorder (Table 1).
2.3. Transcriptional regulation of lysosome biogenesis
TFEB or Transcription factor EB is a key regulator of lysosome biogenesis and function and often referred to as the “master-regulator” of lysosome function. Since its discovery, this transcription factor and its modulation to augment lysosome function, have become the focus of therapeutic strategies for different LSDs as well as neurodegenerative diseases (Napolitano and Ballabio, 2016). A little over a decade ago, a study that began with an in-silico approach (e.g. pattern discovery analysis of the promoter regions of 96 lysosomal genes) lead to the identification of “CLEAR” (‘coordinated lysosome expression and regulation’) elements in promoter regions (Sardiello et al., 2009). This 10 bp palindromic sequence, GTCACGTGAC, was found to be highly enriched in lysosomal genes, as a single copy or in multiple sequences, about 200 bp from their transcription start site. These elements were demonstrated to be binding sites for a basic helix-loop-helix leucine zipper transcription factor, TFEB.
TFEB belongs to the MiTF- TFE family along with MITF, TFE3, TFEC (Napolitano and Ballabio, 2016). At the subcellular level, TFEB localizes to the cytosol, lysosomal surface and the nucleus (Roczniak-Ferguson et al., 2012). TFEB has now been shown to regulate numerous cellular processes relating to lysosome biology including lysosome positioning, lysosome fusion, autophagosome biogenesis, autophagosome–lysosome fusion, (Settembre et al., 2011) as well as lysosomal degradation pathways, including its acidification (Napolitano and Ballabio, 2016). When lysosome function is perturbed, TFEB translocates to the nucleus to promote lysosomal biogenesis. In fact, cells from patients with LSDs such as NPC, show increased nuclear TFEB (Sardiello et al., 2009). Evidence from several laboratories has demonstrated that translocation of TFEB in the nucleus is largely regulated by phosphorylation via nutrient-regulated mTORC1 kinase complex (Roczniak-Ferguson et al., 2012; Settembre et al., 2012). When lysosome function (as well as nutrient levels) are optimal, TFEB translocation to the nucleus is blocked by binding to the lysosomal 14–3-3 protein via mTOR-dependent phosphorylation of Ser 211 (Roczniak-Ferguson et al., 2012). Conversely, nutrient- and mTOR-dependent phosphorylation at S142 and S138 promotes nuclear export of TFEB to the cytosol under conditions of adequate nutrient availability (Napolitano et al., 2018).
TFEB has been implicated in directly controlling lipid catabolism, by regulating expression of lysosomal acid lipase (Palmieri et al., 2011; Settembre and Ballabio, 2014; Thelen and Zoncu, 2017). TFEB also regulates expression of several other genes involved in different steps of lipid degradation, including β-oxidation of fatty acid in mitochondria {Cpt1, carnitine acetyltransferase (Crat), acyl-CoA dehydrogenase} and peroxisomes (CYP4a) (Settembre et al., 2013).
TFEB and other members of this family have also been implicated in various developmental processes including osteoclast differentiation and embryonic stem cell renewal (Napolitano and Ballabio, 2016; Roundy et al., 1999). Thus, the MITF family of transcription factors, by regulating lysosome homeostasis ensure proper functioning of key signalling pathways (including lipid catabolism), and also orchestrate various differentiation and developmental processes.
2.4. Lysosomes as centres for signalling events
Lysosomes are increasingly recognized as a key site of many signalling cascades (Lamming and Bar-Peled, 2019; Perera and Zoncu, 2016). Two of these, nutrient sensing via mTORC1 and calcium homeostasis, are intimately tied with lipid homeostasis (Thelen and Zoncu, 2017).
Lysosomes serve as a platform for the nutrient sensing mTORC1-mediated pathway (Lawrence and Zoncu, 2019). The mTORC1 complex dynamically associates with lysosomes. Under nutrient-rich conditions, mTORC1 promotes growth and anabolic pathways and inhibits catabolic processes such as autophagy through phosphorylation of Unc-51 like autophagy activating kinase (ULK1). mTOR activation requires its recruitment to lysosomes through the hetero-dimeric Rag GTPases. These Rag GTPases are additionally responsible for the recruitment of several other nutrient-responsive molecules such as the Folliculin-FNIP1 complex (Petit et al., 2013) and the transcription factor, TFEB. C9ORF72, a molecule mutated in ALS and frontotemporal dementia (FTD), along with its binding partner SMCR8, is also recruited to lysosomes in a nutrient-dependent manner (Amick et al., 2016).
mTORC1 activation on the surface of lysosomes has important consequences for lipid homeostasis in cells. Its activation promotes de novo synthesis of lipids while also actively suppressing lipid catabolism (Thelen and Zoncu, 2017). It strongly drives expression, trafficking and proteolytic processing of sterol-responsive element-binding proteins (SREBPs), which are master regulators for synthesis of sterols and fatty acids (Düvel et al., 2010). mTOR potently supresses lipid droplet breakdown as well as peripheral β-oxidation. Reciprocally, mTOR activity is also regulated by lipid-derived signals. Phosphoinositides, including PI3P and PI(3,4)P2 have been linked to mTOR regulation (Dong and Czaja, 2011; Thelen and Zoncu, 2017). Recently, cholesterol has been shown to recruit mTOR to the lysosomal surface and this also appears to involve the NPC1 protein (Castellano et al., 2017). This study also suggests impaired mTORC1 activity could contribute to the pathology of NPC (Castellano et al., 2017). Given its effect on lipid metabolism and its reciprocal dependence on lipid homeostasis, the biology of mTORC1 signalling is a critical aspect of lysosomal lipid regulation. Further details of nutrient sensing and mTORC1 signalling pathways at the surface of lysosome, can be found in recent reviews (Lawrence and Zoncu, 2019; Saxton and Sabatini, 2017).
Lysosomal Ca2+ plays an important role in many of the organelle’s functions, including fusion with other organelles such as endosomes, autophagosomes and the plasma membrane. Lysosomal Ca2+ also plays a key role in formation of’contacts’ (discussed in following section) with the ER, which in turn helps fill the lysosome with Ca2+ from the ER (Wang et al., 2017). Lysosomal Ca2+ signalling is also intimately tied up with lipid homeostasis in the organelle. The best characterized lysosomal Ca2+ transporter is mucolipin1 or TRPML1. Activation of TRPML1 by PI(3,5)P2 (Dong et al., 2010) as well as starvation and ROS (Ballabio and Bonifacino, 2020), releases Ca2+ from lysosomes to the cytosol. TRPML1 deficiency causes Mucolipidosis type IV, a LSD characterized by neurodegeneration (Bassi et al., 2000). TRPML1-mediated Ca2+ release from lysosomes is also impaired in NPC disease cells, potentially as a secondary effect of accumulating lipids in the organelle (Hoglinger et al., 2015; Shen et al., 2012). It remains to be seen if TRPML1 activity is altered in other LSDs. TRPML1 mediated Ca2+ release also regulates plasma membrane repair and participates in a feed-back loop with TFEB, mediating TFEB’s ability to promote clearance of substrates in LSDs (Medina et al., 2011). Thus, like with mTORC1 signalling, lysosomal Ca2+ signalling is intimately interwoven with lipid metabolism.
2.5. Interaction of lysosomes with other organelles
Lysosomes undergo homotypic fusion among themselves as well as heterotypic fusion with endosomes, autophagosomes as well as the plasma membrane (Moreau et al., 2013). SNARE proteins are at the centre of these fusion events with lysosomal Ca2+ also playing a key role in the process. Small GTPases ARL8 and Rab7 regulate these SNARE-mediated fusion events between late endosomes and lysosomes as well as homotypic lysosome fusion, through their effector, the hetero hexameric tethering complex, HOPS (Homotypic fusion and vacuolar Protein Sorting) complex (Ballabio and Bonifacino, 2020; Saftig and Klumperman, 2009).
2.6. Lysosome contacts with other organelles
Over the last decade, non-fusogenic interactions of lysosomes with different organelles such as ER, mitochondria, have been characterized (Fig. 2). These contacts appear to have a particularly important role to play in lipid metabolism. These organelle contacts often serve specific functions such as lipid transfer, organelle movement and positioning. While there is no membrane fusion involved, the membranes of the organelles are closely apposed and tethered by specific ‘contact site’ proteins that populate or enrich at these contact sites (Phillips and Voeltz, 2016). Lysosome contact with the ER and its subsequent reorganization around endosomal tubules helps in severing of tubules from endo-lysosomes (Rowland et al., 2014). A major function of ER-lysosome contacts is lipid transfer between these organelles. Cholesterol generated in lysosomal lumen from cholesteryl esters is exported out of lysosomes through concerted action of lysosomal membrane proteins NPC1 and NPC2. They are imported into the ER by the lipid transfer proteins ORP1L and ORP5 and ER resident proteins at contacts, VAPA and VAPB (Dong et al., 2016). The protein STARD3, on lysosomes, interacts with VAP proteins on ER to transfer cholesterol from ER to endo-lysosomes (Wilhelm et al., 2017). Another protein involved in lipid transfer at ER-lysosome contact sites, is VPS13C. The protein is enriched at these organelle contacts and contains a lipid-binding domain and transfers phospholipids (Kumar et al., 2018), although the direction of transfer and its precise function haven’t been identified as yet. One more potential route for lipid transport at ER- lysosome contact sites could involve PDZD8. PDZD8, a resident ER protein, contains a lipid transport SMP module, interacts with GTP-RAB7 on lysosomes, and concentrates at ER-lysosome contact sites (Guillen-Samander et al., 2019). Its precise function in lipid transport is yet to be determined. Lysosomes transfer of free cholesterol to peroxisomes through contacts with these organelles involves lysosomal synaptotagmin 7 and peroxisomal PI(4,5)P2 (Chu et al., 2015).
Fig. 2.
Membrane contact sites between lysosomes and other organelles. Lysosomes form numerous contacts with other organelles. These contact sites are populated by specific proteins, and aid in many different processes. Lysosome contacts with ER play a key role in lipid transfer and metabolism. The small GTPase, Rab7 is involved in lysosome contacts with ER and mitochondria. Lysosomes also form contacts with RNA granules and aid in their transport. Lysosome contacts with the Golgi complex have been implicated in their perinuclear localization.
ER-Lysosome contacts also play a role in lysosome movement and positioning. The ER anchored protein, Protrudin, binds RAB7 and PI3P on lysosomal membranes and promotes the interaction of another PI3P-binding protein, FYCO1 with Kinesin 1 and thus drives lysosome movement to cell periphery (Raiborg et al., 2015). Lysosomal contacts with the Golgi complex, on the other hand, seem to be involved in their peri-nuclear clustering (Starling et al., 2016). Lysosomes also contact mitochondria via RAB7 (Wong et al., 2018). This interaction is counteracted by mitochondrial FIS1 protein through the recruitment of RAB7 GTPase activating protein (GAP), TBC1D15. These lysosome-mitochondria contacts regulate lysosomal RAB7 activity and mark the sites of mitochondrial fission (Wong et al., 2018).
In addition to non-fusogenic contacts with membrane-bound organelles, lysosomes have recently been shown to interact with RNA granules, which are membraneless organelles through the tethering protein, Annexin A11. This hitchhiking of RNA granules on lysosomes, in axons, appears to play a role in local translation of certain mitochondrial proteins (Liao et al., 2019).
Thus, organelle contacts with the lysosome appear to be a critical part of the machinery through which lipid homeostasis is maintained in cells; these sites allow for quick exchange as well as consumption of lipids, when compared to vesicle mediated transport. The growing list of lipid transfer proteins being identified at these contact sites, bears further evidence of the importance of lysosome-other organelle-contacts in maintaining cellular lipid homeostasis.
2.7. Lysosome positioning and lysosomal heterogeneity
The dynamic nature of lysosomes has come to be appreciated over the last decade (Bonifacino and Neefjes, 2017). Lysosomes exhibit microtubule-based movement over long distances. They move from the cell periphery to the perinuclear region (retrograde movement) in a dynein-dependent manner. The movement of perinuclear lysosomes towards the periphery is kinesin-dependent. Lysosome positioning in the cell influences many cellular processes including mTORC1 activity: lysosomes being more peripheral (through overexpression of adaptors/ regulators that promote kinesin-based outward movement) enhance mTORC1 activity while perinuclear clustering reduces it. Likewise, lysosome fusion with autophagosome is also influenced by lysosome positioning, with lysosomal movement towards perinuclear region favoring the fusion events (Korolchuk et al., 2011). Lastly, some heterogeneity in pH and protease content has been observed in lysosomes, based on their position in the cell. Peripheral lysosomes have less acidic pH and lower content of proteases (and thus degradative capacity) (Gowrishankar and Ferguson, 2016; Johnson et al., 2016). This feature was in fact first reported in neurons where axonal and dendritic lysosomes were protease-deficient when compared to lysosomes in soma (Gowrishankar et al., 2015). This lysosome heterogeneity in position and content is a relatively new area of research and the extent of lysosome heterogeneity, in terms of lipid and protein composition, remains to be determined. Experiments that examine the cholesterol and sphingolipid levels of lysosomes in relation to their position (in both non-neuronal and neuronal cells), will shed insight into possible heterogeneity relating to lipid composition of lysosomes. This heterogeneity may also affect how we evaluate the pathology of LSDs. For instance, examination of pH and chloride ion concentrations in lysosomes of NPC-patient fibroblasts revealed that a subset of lysosomes (with high chloride content and low pH) were specifically depleted (Leung et al., 2019). This specific lysosomal population was recovered in the patient fibroblast, after treatment of cells to reduce lipid accumulation. Thus, further elucidation of lysosome heterogeneity could be beneficial to understanding the pathology of various LSDs, as well as designing therapeutic approaches for them.
2.8. Lysosome size: role in physiological function and change in disease states
Lysosomes vary in shape and size depending on cell type (Ballabio and Bonifacino, 2020). Additionally, being highly dynamic, they change their number, size and position in response to various intra and extracellular stimuli. EM-based morphometry analysis has proven to be quite reliable in measuring even moderate changes in organelle ultrastructure. A relatively small change in diameter of lysosomes, results in more drastic effect on surface area and volume these organelles (de Araujo et al., 2020). Organelle size represents a physical constraint determining ratio of membrane surface area to organelle volume: reduction in diameter (and consequently, volume) of endosomal vesicles is shown to reduced intraluminal vesicle budding, which in turn affects cargo sorting and degradation. Lysosome size also affects its dynamics. While the microtubule-based active transport of lysosomes does not seem to be affected by its size, the diffusive component seems to be inversely correlated with organelle size. Increased lysosomal size also appears to negatively impact lysosomal exocytosis. Lysosome size is also altered (often enlarged), due to lipid accumulation in different lysosomal storage disorders (Xu et al., 2014). For instance, NPC and KD deficient cells exhibit enlarged lysosomes (Marshall et al., 2018a; Xu et al., 2012). Lysosomal size is regulated by phospho-inositide composition with lysosomal PI (3,5)P2 being a key regulator (Rutherford et al., 2006). Lysosomal acidification mediated by the vacuolar proton ATPase is another important in regulating vacuole size. In addition to the organelle pH, the V-ATPase has been implicated in lysosomal fission and fusion steps, which will also impact organelle size (Strasser et al., 2011). Loss of acidification and reduced PI (3,5)P2 levels both cause lysosomal enlargement. Thus, a wide variety of conditions including dysregulation of lipid composition, alter lysosomes size.
3. Lysosome dysfunction in neurodegeneration
Neurons, being post-mitotic, are particularly sensitive to build up of damaged organelles and mis-folded proteins arising from lysosome dysfunction. Thus, lysosome dysfunction is an important component of the pathology associated with many neurodegenerative diseases such as AD, PD, ALS and FTD. Abnormal accumulation of protease-deficient lysosomes in swollen axons around amyloid plaques is observed in human AD as well as mouse models of the disease (Gowrishankar et al.,2015). Autophagic dysfunction is also a feature of both early and late stages of AD pathology (Nixon and Yang, 2011). AD patients with mutations in presenilin (PSEN1) exhibit defects in autophagic and lysosome function, which is attributed to defective lysosome acidification and dysregulated Ca2+ homeostasis (Lee et al., 2015). Likewise, the β-CTF studies using mouse models of AD indicate that defects in axonal lysosome transport contribute to AD pathology (Rodrigues et al., 2012). In recent years, many lysosome-autophagy genes have been identified as risk factors associated with PD. For example, heterozygous mutations in the lysosomal enzyme glucosylceramidase (GBA) increase risk for PD while homozygous mutations cause an LSD. GBA mutations leads to reduced lysosome function and contributes to synuclein accumulation, thus linking lysosomes, lipid metabolism and PD pathology (Dodge, 2017). In 2017, met-analysis of genome-wide association studies (GWAS) identified 17 new risk loci (Chang et al., 2017). This included several genes associate with lysosome biology in addition to GBA. These are TMEM175 (recently shown to encode a potassium channel that could regulate lysosome function), CTSB (Cathepsin B, a lysosomal cysteine protease), and GALC (Galatosylceramidase), a gene also signalled as a risk for PD, (Abdelkarim et al., 2018; Marshall et al., 2018b; Smith et al., 2014). A PD risk allele results in reduced levels of CTSB mRNA. CTSB is involved in degrading soluble and membrane-bound α-synuclein in mice. Viral over expression of CTSB also helps clear amyloid plaques in AD mouse models (Sun et al., 2008).
In addition to these links with lysosomes, as described in PD, many of these neurodegenerative diseases also exhibit altered lipid metabolism. In AD, for instance, the most common genetic risk factor, ApoE, is involved in lipid transport and metabolism (Chew et al., 2020). The involvement of lipids in AD pathology has been reviewed extensively, elsewhere (Chew et al., 2020). Likewise, age- and dose-dependent changes in glycolipid cell metabolism can lead to PD (Hallett et al., 2019). Thus, understanding pathologies of other neurodegenerative diseases that relate to lipid metabolism could yield important insight into LSDs.
3.1. Lysosomal storage disorders or LSDs
LSDs are a classical example of disorders cause by lysosome dysfunction. Currently, they include about 40 different monogenic diseases characterized by multi-system phenotypes and often, early-onset neurodegeneration (Ballabio and Gieselmann, 2009). They are caused by mutations or loss of function of lysosomal resident proteins or non-lysosomal proteins that regulate lysosomal degradation leading to accumulation of undigested material (lipofuscin) (Platt, 2018). These can be due to mutations leading to loss of soluble hydrolases, in which case there is accumulation of a specific substrate. In others, such as loss of function of TRPML1, the Ca2+ transporter, there are broader cellular consequences to the disease (Bassi et al., 2000). Certain cases of LSDs arise from mutations in genes that are involved in sorting, transport or post-translational modification of lysosomal enzymes. In addition to pathology arising from loss of the enzyme function, studies have demonstrated that there can be secondary pathological consequences. For instance, autophagosome-lysosome fusion is affected in many LSDs, often due to aberrant cholesterol accumulation. Another key secondary consequence is altered mTORC1 activity. While in the case of NPC, there is increased mTORC1 activity (Bartolomeo et al., 2017), fly models of Gaucher’s disease (GD) show reduced mTORC1 activity (Kinghorn et al., 2016. Table 1 provides a brief description of some of the major LSDs and their symptoms. We further discuss three of these lysosomal storage diseases, NPC, MLD, and KD. Detailed description of other LSDs, their cellular phenotypes have been reviewed elsewhere (Platt, 2014; Platt, 2018; Platt et al., 2012; Platt et al., 2018).
4. Lipidosis affecting the transport of cholesterol: Niemann-Pick type C
4.1. Synthesis and transport
Cholesterol metabolism is a highly regulated process that involves several organelles across different organs. The biosynthesis begins with acetyl-CoA through the mevalonate pathway with squalene synthesis as the critical point to sterol production and ultimately the reduction of 7-dehydrocholesterol to cholesterol as the final product. Post-squalene processes occur in the endoplasmic reticulum (ER) through several enzymatic steps. Cholesterol levels are tightly regulated with contributions from dietary intake as well as de novo synthesis. De novo synthesis is primarily controlled by HMG-CoA regulation in a feedback inhibition manner. However, additional roles of the membrane-bound transcription factor, sterol-regulated element binding protein (SREBP) further contribute to maintaining cholesterol levels. Upon synthesis, cholesterol is esterified by the appropriate enzymes and transport is carried out via lipoprotein particles. Specialized cholesterol transporters can also participate in the movement of cholesterol. In addition to structural and signalling roles, cholesterol also serves as a precursor for bile acids and other steroid hormones. The general concepts of cholesterol biosynthesis have been recently reviewed including considerations for the brain (Alphonse and Jones, 2016; Jin et al., 2019; Zhang and Liu, 2015). Given the diverse roles of cholesterol in cellular compartments, the movement as well as the recycling process of cholesterol must be highly specialized. Important for the topic of this article, we focus on post-lysosomal cholesterol movement and how its defect can alter cell function. Various routes of cholesterol transport have been identified. Lange et al., reported that LDL-derived cholesterol trafficked through lysosomes does not directly traffic to the ER but rather to the plasma membrane first (Lange et al., 1997). However, reports of direct transfer to the ER from the lysosome has been presented by other groups (Neufeld et al., 1996) including one report which utilizes a pharmacological model of impaired lysosomal cholesterol trafficking (Underwood et al., 1996). Regardless, of these divergent findings it is well regarded that cholesterol trafficking through the endo-lysosomal system is critical and multiple destinations are feasible which may depend on the source of cholesterol (Storch and Cheruku, 2005). Interestingly, a recent report investigating lysosomal-derived cholesterol indicated that phosphatidylserine is needed for cholesterol trafficking to the plasma membrane then the ER (Trinh et al., 2020), highlighting the complexity of cholesterol metabolism. The sophistication of cholesterol synthesis, transport and metabolism highlights the potential impact on cellular function and human disease when this important lipid is altered.
4.2. Cholesterol and Niemann-Pick type C disease
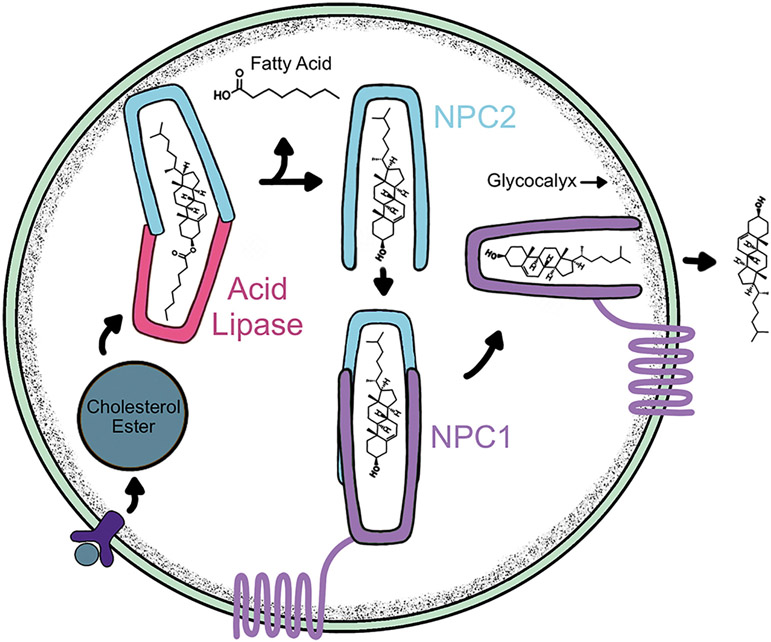
Disruption in cholesterol homeostasis has been implicated in a variety of human diseases. For example, impaired cholesterol biosynthesis causes Lathosterolosis (SC5D mutations) and Smith-Lemli-Opitz Syndrome (DHCR7 mutations) (Porter and Herman, 2011). Tangier disease (OMIM: 205400) arises from mutations of the ATP binding cassette transporter, ABCA1, which impairs transport and release of cholesterol results in low levels of high-density lipoproteins and therefore accumulation of cholesterol (Fitzgerald et al., 2010). Regarding lysosomes and cholesterol, the most classical example is Niemann-Pick Type C (NPC) disease (Vanier and Millat, 2003). NPC arises from mis-trafficking of cholesterol through the endo-lysosomal system and is caused by mutations of either the NPC cholesterol transporters 1 or 2 (NPC1 or NPC2) genes, which work in tandem to transport unesterified cholesterol through the lysosome (Cologna and Rosenhouse-Dantsker, 2019; Kwon et al., 2009; Li et al., 2017; Pfeffer, 2019) (Fig. 3).
Fig. 3.
The lysosome serves as a recycling hub for cholesterol. Receptor-mediated uptake of lipoproteins results in binding of cholesterol by the NPC2 lysosomal protein and the fatty acid is cleaved by acid lipase. The free cholesterol is then handed off to the large, lysosomal membrane protein, NPC1. Cholesterol is then egressed out of the lysosome to the ER or other destinations.
As an autosomal recessive LSD, NPC has an estimated incidence of 1:100000 live births (Wassif et al., 2016) (Table 1). Approximately 95% of NPC diagnoses arise from NPC1 mutations and the clinical phenotype is broad. Impaired cholesterol trafficking leads to accumulation of cholesterol and multi-organ dysfunction. Examples of alterations in NPC laboratory models and patients include oxidative stress (Fu et al., 2010; Klein et al., 2011), mitochondrial defects (Kennedy et al., 2014) impaired autophagy (Guo et al., 2016; Pacheco et al., 2007; Sarkar et al., 2013), and neuroinflammation (Cologna et al., 2014), among others. Several recent review articles have discussed NPC disease both in terms of basic findings and clinical perspectives (Newton et al., 2018; Papandreou and Gissen, 2016; Wheeler and Sillence, 2019).
Over the past five years, the NPC research community has made several important contributions and findings to our understanding of the disease. Many of these reports are built upon previous work to investigate potential drug therapies for NPC patients including the glycosphingolipid inhibitor Miglustat (Zavesca ®) which is not FDA-approved but is approved in other countries (Lachmann, 2003; Lachmann et al., 2004; Patterson et al., 2007). Currently, the most promising therapy is 2-hydroxypropyl-beta-cyclodextrin, which is known to bind cholesterol molecules (De Caprio et al., 1992). However, the direct mechanism of action in NPC is still an active area of research. In small animal studies, 2-hydroxypropyl-beta-cyclodextrin has been shown to ameliorate cholesterol storage, reduce clinical signs and extend lifespan (Davidson et al., 2009). Using a feline model of NPC, Vite and co-workers showed that direct central nervous system administration of 2-hydroxypropyl-beta-cyclodextrin results in improved Purkinje cell survival and lifespan (Vite et al., 2015).
Clinically, reports have indicated that 2-hydroxypropyl-beta-cyclodextrin is safe in single patient (Maarup et al., 2015) and multi-patient trials (Berry-Kravis et al., 2018; Garcia-Robles et al., 2016; Ory et al.,2017. Several groups are investigating the mechanism of action of 2-hydroxypropyl-beta-cyclodextrin. For example, 2-hydroxypropyl-beta-cyclodextrin has been reported to affect synaptic transmission (Frech et al., 2015), to enhance endo-lysosome secretion (Vacca et al., 2019), and upregulates LAMP1 levels which may assist in cholesterol efflux in the absence of functional NPC1 protein (Singhal et al., 2018). Additionally, 2-hydroxypropyl-beta-cyclodextrin reduces microglial activation (Cougnoux et al., 2018), restores APMA receptors function (Feng et al., 2019) and promotes re-distribution of cholesterol (Feltes et al., 2020). Additionally, 2-hydroxypropyl-beta-cyclodextrin has been shown to promote cholesterol secretion which is dependent upon the Mucolipin-1 (MCOLN1) cation channel (Vacca et al., 2019).
Continued efforts have been focused on biomarker discovery in NPC (Bradbury et al., 2016; Giese et al., 2015; Mazzacuva et al., 2016; Rauniyar et al., 2015) and validation in patients (Sidhu et al., 2015; Sidhu et al., 2019; Wos et al., 2016). Examples range from untargeted differential proteomic (Cologna et al., 2012; Pergande et al., 2019a; Pergande et al., 2019c) and lipidomic analysis (Fan et al., 2013; Pergande et al., 2019b). Examples of biomarkers that have been utilized beyond the research laboratory include bile acid monitoring in newborn screening (Jiang et al., 2016), serum cholesterol oxidation products for diagnostics (Jiang et al., 2011) and current monitoring of serum 24(S)-hydroxycholesterol as well as cerebrospinal fluid fatty acid binding protein 3 and Calbindin D protein biomarkers in drug trials (Ory et al., 2017). It is expected that these types of studies will continue to further understand the roles of different mutations, experimental treatments, and modulation of known alterations in NPC.
In 2015, the Ory laboratory developed the homozygous I1061T mouse model (Praggastis et al., 2015), which has been critical for the NPC community. This mouse model represents the most common mutation seen in NPC patients and provides an additional resource beyond the other genetic animal models available (as reviewed in (Fog and Kirkegaard, 2019)). Biochemically and phenotypically the I1061T NPC1 mouse model mimics the previously more commonly used null nih model; however, the onset of symptoms and lifespan of mutant mice are slightly longer in this model, which is on a different genetic background than the null model. The same group had previously shown that the I1061T mutation produces a functional, yet, rapidly degraded version of the NPC1 protein (Gelsthorpe et al., 2008). Moreover, Schultz et al. (Schultz et al., 2018) have shown that this degradation is carried out by selective ER autophagy and requires the autophagy receptor, FAM134B. Although the I1061T point mutation is the most common, hundreds of clinically relevant mutations have been reported including evidence of mutational differences in protein phenotype and function (Shammas et al., 2019) which further highlights the need for additional studies to understand the consequences of specific mutations.
The NPC research field has many new and exciting findings that are on the horizon for making lasting impacts on the patients and research community. In terms of developing therapies for NPC patients, perhaps the next emerging option is gene therapy (Chandler et al., 2017). Direct brain administration of AAV9-human NPC1 shows overexpression of the protein and lysosomal localization in wild-type mice, and shows improved phenotypic characteristics and biochemical markers in mutant mice (Hughes et al., 2018). Similar findings show single injections also result in Purkinje cell survival in mutant mice and increase lifespan of these animals (Xie et al., 2017). Membrane contact sites have also been an emerging research theme in the NPC community. Membrane contact sites allow for inter-organelle transport of materials (as reviewed: (Prinz et al., 2020)). In the context of NPC, a recent study provides evidence that NPC1 plays a tethering role in ER-endocytic and ER-lysosome contacts namely with Gramd1b (Hoglinger et al., 2019). However, in the absence of NPC1, membrane contact sites between lysosomes and mitochondria are observed (Lim et al., 2019). Finally, the oxysterol binding protein, ORP1L, has been shown to facilitate cholesterol transfer between ER-lysosome membranes and that this transport is increased in cells lacking NPC1 (Rocha et al., 2009). These findings are further complemented by a recent report of ER-lysosome contact sites facilitating cholesterol transport via the oxysterol binding protein 1, which can enable mTORC1 signalling through cholesterol sensing by the mTORC1 protein (Lim et al., 2019). Together, these new reports of membrane contact sites and their importance in NPC disease provide new insights to understanding the pathophysiology of the disorder and to develop new therapeutic strategies.
Ca2+ is a critical secondary messenger and participates in an array of signalling processes within the cell. Suggestions for alterations in Ca2+ levels, distributions and signalling were implicated in NPC in the 1980s and 1990s specifically looking at phospholipase c activity (Beaudet et al., 1980) and Ca2+ channel function in fibroblasts (Yamamoto et al., 1994). Over the next several decades, implications of Ca2+ dysfunction were included in several reports; however in 2008, Lloyd-Evans et al., suggested that NPC includes lysosomal Ca2+ defects (Lloyd-Evans et al., 2008). Subsequent findings show Ca2+ release mediated by TRPML-1 from lysosomes is impaired in NPC (Shen et al., 2012). Alterations have also been shown in astrocytes (Saez et al., 2013), iPSC derived neurons (Rabenstein et al., 2017) and primary neuron cultures (Tiscione et al., 2019). The critical role of Ca2+ homeostasis may indicate that understanding the causes and effects of Ca2+ dysfunction is critical for development treatment options for NPC.
While much of the NPC research has focused on the progressive neurodegeneration and specifically loss of Purkinje neurons, it is also important to recognize the presence of demyelination in animal models (Qiao et al., 2018) and patients (Davies-Thompson et al., 2016). Perhaps not surprising, the dysregulation of cholesterol synthesis and trafficking in NPC directly affects oligodendrocyte function and myelin maintenance. Reports of demyelination show myelin damage in the NPC corpus collosum (German et al., 2002; Yang et al., 2019). Interestingly, deletion of neuronal NPC1 results in impaired maturation of oligodendrocytes while loss of NPC1 in oligodendrocytes leads to delayed myelination (Yu and Lieberman, 2013). Importantly studies using a feline model of NPC showed decreased myelin thickness and reduced axon diameter in support of mouse model studies (Bagel et al., 2013). These reports demonstrate the importance of considering neuron-glial cross talk as well as the role of impaired cholesterol trafficking in demyelination. It is likely that the NPC field will see a shift in focus from neuron-centric to a more holistic and glial inclusive disease.
4.3. Deregulation of signalling cellular function by cholesterol
The direct defect of cholesterol trafficking in NPC provides evidence to the critical relationship in cholesterol metabolism and signalling in neurodegenerative disorders (see review (Arenas et al., 2017)). Beyond NPC, impairments in cholesterol metabolism and cholesterol mediated processes have been reported in several neurological diseases (Fig. 4). Just over three decades ago, Ignatius et al., described the connection of upregulated ApoE upon sciatic and optic nerve injury and proposed that this occurs as a repair mechanism (Ignatius et al., 1986). This study was further followed by clarification that upregulation of ApoE was a result of oligodendrocyte synthesis thereby providing lipids for myelin repair (Stoll et al., 1989). The relationship between ApoE and AD has been vastly studied and recently reviewed, perhaps with the most striking link being the APOE4 genotype with pre-disposition to developing AD (Balu et al., 2019; Huang and Mahley, 2014; Tai et al., 2017).
Fig. 4.
Cholesterol in neurodegeneration. Cholesterol is disrupted in many neurodegenerative conditions and plays a role in neuronal cell death, demyelination, microglial activation and synapse dysfunction.
Given the diverse roles of cholesterol in signalling and cellular maintenance it is perhaps not surprising that many similar pathways are altered in neurodegenerative disorders. For example, the PI3K-Akt pathway, which is controlled in cholesterol rich membrane domains is dysregulated in NPC (Bi et al., 2005) and familial AD (Baki et al., 2008). Similarly, a loss of Akt signalling has been reported in familial PD (Yang et al., 2005). Although not performed in a neuronal cell type, Yamauchi et al., demonstrated that PI3K-Akt-mTOR1 played a role in SREBP regulation (Yamauchi et al., 2011). Akt and cholesterol biosynthesis are intimately intertwined with regard to myelin maintenance (Mathews and Appel, 2016).
Another critical cholesterol-involved signalling pathway is the Hedgehog pathway. In terms of NPC research, this area has not been extensively studied. The Scott group showed that Shh is produced by cerebellar Purkinje neurons and that granule cell precursor division can be regulated by Shh (Wechsler-Reya and Scott, 1999). The progressive loss of Purkinje cells in NPC may have an effect on gradual cell populations in animal models and patients; however, a specific link has not been shown to our knowledge. The protein Patched,is a cholesterol binding protein and negative regulator of the Hedgehog signalling pathway. In the absence of Patched, neurodegeneration results and Patched has been shown to be decreased in NPC models (Formichi et al., 2018) as well as impairment of primary cilia (Canterini et al., 2017). Similar to NPC, the role of Shh and AD pathophysiology is also limited however, there is some evidence that altered Shh signalling is present via Patched deregulation in AD implicating a critical role for cholesterol homeostasis (He et al., 2014).
It is also important to add the critical role of cholesterol in canonical Wnt signalling (Sheng et al., 2014), downstream to PI3K/Akt. Related to LSDs, defects in Wnt have been reported in iPSC cells of NPC (Efthymiou et al., 2015), GD (Awad et al., 2017; Zancan et al., 2015) but not in KD or other lipidoses. Significant effort has been put towards linking Wnt signalling in AD. In a study of familial AD, presilin 1 mutations result in trafficking of beta-catenin and downstream Wnt signalling (Nishimura et al., 1999). Increased phospho-beta-catenin is present in AD hippocampal pyramidal neurons and links proteasome defects with Wnt signalling and AD (Ghanevati and Miller, 2005). In fact, multiple groups have led efforts to develop treatments targeting Wnt for AD including investigating fluoxetine (Huang et al., 2018), sodium selenate (Jin et al., 2017) and ethosuximide (Tiwari et al., 2015) among others. These reports all point to similarities in the alterations among neurodegenerative disorders and highlight the need for additional work to understand cholesterol signalling among the many LSDs with neurodegenerative phenotypes.
5. Lipidoses of galactolipids: metachromatic leukodystrophy and Krabbe’s disease
5.1. Metabolism of galactosylceramides and sulfated galactosylceramides
Sphingolipids are synthesized in the endoplasmic reticulum and the Golgi apparatus, through the production of 3-ketodihydrosphingosine by serine palmitoyl-transferase activity on serine and palmitoyl CoA (Pralhada Rao et al., 2013). This precursor leads to the formation of ceramide, a key component of these complex sphingolipids. Ceramide is composed by sphingosine and fatty acyl chains and glycosylation of ceramides by ceramide glycosyltransferases renders galactosylceramides and glucosylceramides, important lipids in myelin and neuronal membranes. An additional pathway for the production of ceramide is mediated by the hydrolysis of membrane-bound sphingomyelin by the activity of neutral and acid sphingomyelinases (Fig. 5). Sulfatides, also known as 3-O-sulfogalactosylceramide, SM4, or sulfated galactocerebrosides, are a heterogenous populations of lipids, characterized by the presence of a negative charged sulfate group. Sulfates are added to galactosylceramides via the activity of 3′-phosphoadenosine-5′-phosphosulfate:cerebroside sulfotransferase (CST) enzyme, utilizing 3’phosphoadenosine-5′-phosphoslfate (PAPS) as a donor. Due to availability of multiple forms of acyl fatty acid chains (e.g. short, long, saturated, unsaturated, hydroxylated), galactosylceramides and sulfatides show multiple isoforms (Marbois et al., 2000; Svennerholm and Stallberg-Stenhagen, 1968). For example, galactosylceramides and sulfatides show more than 30 isoforms or species in the mouse and human brain.
Fig. 5.
Main pathways of the glycosphingolipid metabolism.
Glycosphingolipids are degraded by specific hydrolases in the lysosome. Glucosylceramides and their lysosphingolipid glucosylsphingosine (glucopsychosine) variant are degraded by GBA while galactosylceramides and their lysosphingolipid variant galactosylsphingosine (psychosine) are degraded by galactosylceramidase (GALC). Sulfatides are largely degraded by arylsulfatase A (ARSA). Mutations in the GBA, GALC and ARSA genes are the genetic cause for GD, KD and MLD, respectively, lysosomal deficiencies with serious neurological complications. Deficiency in sphingomyelinase activity is the cause of Niemann-Pick type A and B diseases.
6. Metachromatic leukodystrophy: a sulfatide lysosomal storage disease
6.1. Genetics and pathophysiology
Metachromatic Leukodystrophy (MLD) is an autosomal recessive inherited metabolic disease caused by mutations in the ARSA gene (Gieselmann et al., 1998). Most MLD patients show early symptoms but late-infantile, juvenile and adult forms are also present. The incidence is ~1:40,000 births, being the infantile form the most frequent (Gustavson and Hagberg, 1971) (Table 1). Infantile MLD patients present with early cognitive and locomotor dysfunction, demyelination and neurodegeneration, with premature death. MLD is still incurable although hematopoietic stem cell transplantation from healthy donors provides some control of the disease when administered in pre-symptomatic patients (Biffi et al., 2013; Boucher et al., 2015; Cartier and Aubourg, 2008; Groeschel et al., 2016). Additionally, recent advances in gene therapy using lentiviruses and adeno-associated viruses (AAV) are promising (Miyake et al., 2014; Piguet et al., 2012; Rosenberg et al., 2016).
The accepted pathogenic mechanism involves the progressive accumulation of sulfatides in oligodendrocytes, causing cell dysfunction and myelin loss (Gieselmann, 2008). However, the finding of sulfatides in non-myelin membranes such as neuronal membranes (Isaac et al., 2006) strongly underlines the possibility that accumulation of sulfatides impacts more cellular systems. The involvement of sulfatides in additional non-myelin deficits may be better appreciated in late-onset forms of the disease. For example, late-infantile patients showed decreased cerebral grey matter volume compared with age-matched controls (Groeschel et al., 2012). Adult MLD often presents with cognitive psychiatric disabilities years before major motor deficiency is noted (Kolodny and Fluharty, 1995; Shapiro et al., 1994). Interestingly overexpression of CGT or CST enzymes in neurons using the Thy1.2 promoter showed that increasing sulfatides levels in neurons lead to lethal audiogenic seizures in mice (van Zyl et al., 2010), a feature also observed in MLD patients and the MLD mouse model (Hess et al., 1996). This implicates deficits in sulfatide metabolism in myelin and non-myelin lead to variegated pathogenic responses. Furthermore, recent work has shown signs of dysfunction in MLD microglia even in areas where myelin lesions are not evident (Bergner et al., 2019). These findings also reveal that changes in sulfatide homeostasis (Safaiyan et al., 2016) are pathogenic for microglia much earlier than previously considered. This new aspect of MLD pathophysiology indicates a central role for microglia in disease progression and is aligned with growing evidence for similar roles for this immune cell type in a number of other LSD.
6.2. Sulfatides in oligodendrocyte differentiation and myelination
Although sulfatides are enriched the plasma membrane of most cells (Inui et al., 1988) and exert pleiotropic functions (for a complete review please read (Takahashi and Suzuki, 2012)), their biological function(s) remain largely unknown. When we think about the function of lipids in biological membranes, the first role that comes into consideration is as molecules that provide structural scaffolding to membrane associated proteins. Expectedly, sulfatides, like many other sphingolipids, are largely located on the outer leaflet of the plasma membrane. There is evidence that sulfatides may act as “receptors” for the interaction with extracellular molecules. One of these interactions is that with laminin and tenascin-R to promote signalling through integrin and dystroglycan receptors (Li et al., 2005). Interference of this interaction using antibodies anti-sulfatides blocks downstream signalling causing a negative regulation of oligodendrocyte maturation (Baron et al., 2014).
In the nervous system, sulfatides are crucial components of myelin, a specialized membrane produced centrally by oligodendrocytes and peripherally by Schwann cells. Sulfatides constitute one fifth of all galactosylceramides and 4–7% of the dry weight of lipids in myelin (Norton and Cammer, 1984). Reports have shown that, although in low quantities, neurons, astrocytes and neural precursors also synthesize sulfatides (Isaac et al., 2006; Pituch et al., 2015).
The role of sulfatides in myelination was mainly studied using knockout (KO) mice null for CST (Honke, 2013; Ishibashi et al., 2002; Takano et al., 2012) or ARSA (Hess et al., 1996). Deficiency of sulfatides in CST KO mice revealed sulfatides’ contribution to the stability of myelin, particularly that of the paranodal regions. Interestingly, while CST KO mice do not show any major cellular problem (Honke, 2013; Ishibashi et al., 2002; Takano et al., 2012), increased number of oligodendrocytes in the spinal cord are detected (Shroff et al., 2009), apparently due to an increased survival. These findings supported a theory that sulfatides are negative regulators of oligodendrocyte differentiation (Bansal et al., 1999; Hirahara et al., 2004).
On the other hand, the ARSA-KO mouse has increased levels of total sulfatides (Hess et al., 1996) but does not completely recapitulate the infantile human disease but shows mild demyelination and neurodegeneration. Interestingly, overexpression of sulfatides in oligodendrocytes of the ARSA KO via CST genetic manipulation causes demyelination and neurological signs (Ramakrishnan et al., 2007). This finding underscores the importance of lipid levels in triggering sufficient myelin instability to cause demyelination.
Although long acyl chain (>C18) sulfatides are the main isoforms expressed in mature myelinating cells (supporting their role(s) in myelination), the finding of short acyl chain (<C18) sulfatides at earlier premyelinating developmental times underlines additional roles for these lipids. Sulfatides are present during embryonic development in the spinal cord (colocalizing with Olig-2+ oligodendrocyte progenitors, (Hirahara et al., 2017)), sciatic nerves (Li et al., 2005) and telencephalic neural precursor cells (Pituch et al., 2015) well before myelination. The expression of short acyl chain sulfatides before myelin synthesis also suggests additional functions of sulfatides in precursor cells, which may not involve a structural role in myelin membranes but rather, related to sulfatides’ association with lipid rafts (see below).
6.3. Sulfatides in neurons and immune cells
Few studies have addressed the role of sulfatides in neurons. Sulfatides with short acyl fatty chains are present in neurons and astrocytes and are mainly associated with endosomal vesicles. Previous studies have shown that sulfatides inhibit neurite outgrowth in cultured neurons via Rho signalling (Winzeler et al., 2011) and if over-expressed in neurons of the ARSA null mouse, sulfatides facilitate motor and cortical hyperexcitability (Eckhardt et al., 2007). Sulfatides are also known for their involvement in immune cell signalling. Blomquist demonstrated that although many sulfatides have promiscuous effects on antigen presenting cells (APC), lysosulfatides and the C24:1 sulfatide isoform induced the strongest stimulation (Blomqvist et al., 2009). Sulfatides also stimulate type II NK T-cells in the content of MIH class I (Jeon et al., 2008). Although this study was done under in vitro conditions, it clearly demonstrated that sulfatides induce the production of inflammatory mediators in microglia and astrocytes. Sulfatides but not galactosylceramides can trigger the phosphorylation of p38, ERK and JNK via a L-Selectin dependent-mechanism (Jeon et al., 2008). In view that sulfatides are enriched in myelin, these finding underlines a possible role for these lipids during demyelination and immune responses in the brain. In fact, type II NK T-cells recognize myelin derived sulfatides during experimental allergic encephalomyelitis (EAE) (Maricic et al., 2014), while activation of sulfatide reactive type II NK T-cells by sulfatides reduces EAE (Jahng et al., 2004).
Likely related to non-myelin functions, sulfatides have been found dysregulated in neurodegenerative diseases like PD (Fabelo et al., 2011), AD (Haughey, 2010) and Multiple Sclerosis (Marbois et al., 2000; Moyano et al., 2016; Moyano et al., 2013). Whether this reflects a generalized and non-specific response to brain damage or due to specific sulfatide functions beside those in myelination remains unclear. Together, these findings reinforce the pleotropic roles that sulfatides have in the nervous system, and that a better understanding of the pathophysiological mechanisms associated with this important class of sphingolipids will contribute to understand pathogenic mechanisms in multiple neurodegenerative conditions.
7. Krabbe’s disease: a psychosine lysosomal storage disease
7.1. Genetics and pathophysiology
Patients with a diffuse and lethal sclerosis of the brain were first described by Knud Haraldsen Krabbe, a Danish neurologist in 1916 (Krabbe, 1916). KD (also known as globoid cell leukodystrophy or GLD) is an autosomal recessive condition caused by mutations in the gene encoding for galactosylceramidase (GALC). GALC is a lysosomal hydrolase that catalyses the removal of galactose from psychosine and galactosyl-ceramides (Table 1). GALC deficiency leads to the progressive accumulation of psychosine in multiple organs, but particularly in the nervous system. The high levels of psychosine elicit a toxic environment for myelinating cells, promoting diffuse global demyelination, accompanied by gliosis, and neuronal dysfunction. Most Krabbe patients are infants with a rapid and invariably fatal course, carrying the 502 T/del mutation, which removes a 30 kb segment in the 3′ region of the gene (Wenger et al., 2019). The disease can manifest as early infantile (most cases), juvenile or adult onset forms, with over 110 mutations described (Wenger et al., 2019). The disease affects both sexes indistinctly and generally starts with hyperirritability, limb stiffness, and continues with rapid and severe motor and mental deterioration and early death (Suzuki, 2003). Although hematopoietic stem cell (HSC) transplantation (HSCT) is a well-established protocol for hematopoietic replacement, which slows disease progression when administered in presymptomatic Krabbe babies, it is compounded with significant morbidity and mortality. Although HSCT clearly protracts disease, HSCT-patients recur with significant paralysis and death during the teen years (Escolar et al., 2005; Galbiati et al., 2009; Krivit et al., 1998; Luzi et al., 2005; Reddy et al., 2011; Yagi et al., 2005). Promising gene therapy pre-clinical trials using AAV vectors have shown their efficacy to better control disease in animal models, and underline the possibility for combination with HSCT (Bradbury et al., 2018; Karumuthil-Melethil et al., 2016; Marshall et al., 2018a; Qin et al., 2012; Reddy et al., 2011). However, several key aspects of the pathogenic mechanisms in KD remain unanswered, which has limited advancing a cure for patients.
7.2. Signalling and mechanisms targeted by psychosine
Psychosine is a sphingolipid with known pleiotropic pathogenic characteristics. Being a myelin disease, it is not surprising that psychosine targets the activation of pro-apoptotic pathways in oligodendrocytes and Schwann cells, which leads to cell death and presumably to myelin loss. Numerous studies have used using a variety of cell model systems to determine how psychosine induces apoptosis of oligodendrocytes and various mechanisms have been identified. Psychosine interferes with mitochondrial potential, activation of caspases including caspase 3 and 9, release of toxic levels of cytochrome C, aberrant signalling through AMPK, phospholipase A2, connexin 43, extracellular calcium and membrane damage (Cho et al., 1997; Giri et al., 2008; Giri et al., 2006; Graziano et al., 2016; Haq et al., 2003; Hawkins-Salsbury et al., 2013; Inamura et al., 2018; Smith et al., 2011; Voccoli et al., 2014; White et al., 2011; White et al., 2009; Won et al., 2013; Zaka and Wenger, 2004). In addition to these, psychosine was shown to promote inflammatory responses in glial cells, including microglia, astrocytes and macrophages (Claycomb et al., 2014; Ijichi et al., 2013; Kondo et al., 2011; LeVine and Brown, 1997; Ohno et al., 1993; Snook et al., 2014).
Although psychosine has historically been studied only in myelinating cells, various studies demonstrated that neurons are particularly sensitive to this lipid. For example, Cantuti-Castelvetri and coll. Found that axons in Krabbe’s disease suffer of axolemmal swelling, and undergo rapid atrophy with segmentation fitting the dying back neuropathy model (Castelvetri et al., 2011; Smith et al., 2011). Further studies demonstrated that axonopathy was accompanied by exacerbated psychosine-sensitive phosphatase activity, primarily via PPI and PP2A, promoting abnormal levels of dephosphorylated neurofilaments, which likely contributes to the thinning of axons diameter (Duchen et al.,1980). Axonopathy and dying back neuropathy are highly linked to how the axon is capable to sustain healthy communication between the neuronal body and the axonal terminal. Optimal rates of axonal transport are key to support this communication and factors that reduce axonal transport inevitably will undermine neuronal connections. In this regard, Cantuti-Castelvetri and coll. were the first to demonstrate that psychosine is sufficient to reduce anterograde and retrograde axonal transport modes (Cantuti Castelvetri et al., 2013), which established the basis of dying-back neuropathy in KD. Interestingly, the mechanism by which psychosine facilitated this pathogenic response in axonal transport was not mediated by myelin, plasma membrane or even neuronal and/or oligodendroglial components. By using axolemma-free preparations of squid axoplasm, these authors demonstrated that psychosine is sufficient to stimulate a PP1-GSK3β aberrant phosphorylation of motor proteins in the axon, which leads to reduced transport rates (Cantuti Castelvetri et al., 2013). These findings underlined the possibility that some of the pathogenic responses of psychosine might not need a direct involvement of membranes but rather, intermolecular interactions. In this regard, psychosine was found to directly bind to negatively charged amino-acids in the carboxi-terminus of α-synuclein (Abdelkarim et al., 2018), promoting the aberrant formation of α-synuclein aggregates in neurons of the Krabbe brain (Smith et al., 2014).
Although many additional pathogenic processes have been described in KD, with the exception of the study of Abdelkarim and coll. demonstrating a direct interaction between psychosine and α-synuclein (Abdelkarim et al., 2018), all other studies reported to date have not been able to answer a common question: how do membrane-bound glycosphingolipids such as psychosine operate to trigger cellular and signalling responses which occur in the cytoplasm? This question, shared in other lipidoses such as Niemann-Pick types A, B, C, MLD, and GD, is further discussed below.
8. Lipid rafts: membrane tethered signalosomes affected in lipidoses
Emerging evidence has shown that most biological membranes have the property of reorganizing specific sets of lipids into limited nanometric domains known as lipid rafts (Carquin et al., 2016; Lingwood and Simons, 2010; Simons and Ikonen, 1997; Singer and Nicolson, 1972) ( Fig. 6A). Lipid rafts are small (10–250 nm in diameter) dynamic platforms that operate by enabling transient or stable associations between specific lipids including cholesterol and several classes of sphingolipids and raft-associated proteins, including receptors, scaffold proteins and signalling intermediates. Lipid raft domains may be flat but they may also fold contributing to the formation of caveola.
Fig. 6.
Models of signalling disruption in lipidoses. A) Under physiological conditions, cholesterol and glycosphingolipids tend to coalesce in more rigid lipid raft domains, which provide platforms where multiple components participating in signalling may interact with optimal efficiency. B) In lipidoses, the abnormal accumulation of glycosphingolipids or cholesterol in lipid rafts alter the fluidity and lateral mobility of raft-associated components, reducing or blocking the transduction of cell signalling. In this example, even though all components participating in the IGF pathway are essentially not altered, deformation of the raft interferes with their proper interaction, blocking downstream activation of AKT. C) A complementary model to explain dysfunctional signalling in lipidoses involves the shedding or vesiculation of membranes leading to the loss of key components and blockage of the signal.
There are three major lipid groups largely present in all plasma membranes: 1) cholesterol, which is a non-polar sterol molecule inserted within the membrane bilayer, exposing a single hydroxyl group above the membrane surface; 2) glycerophospholipids (i.e. phosphatidylcholine) composed by saturated/unsaturated fatty acyl chains and a polar head-group and 3) sphingolipids composed by a hydrophobic sphingoid base (sphingosine), in most cases acylated with fatty acids to form ceramide, which is linked to head-groups such as choline (sphingomyelin), sugars (galactosylceramide, glucosylceramide) or even more complex and larger head-groups (i.e. sulfatides; gangliosides). Many of these traditional glycosphingolipids have also lysophingolipid versions, composed by sphingosine and the corresponding sugar (i.e. galactosylsphingosine or psychosine). Rafts are important for membrane curvature. The shapes of lipids significantly influence how much a membrane can bend, a fundamental property for maintaining cell shape, endocytosis and exocytosis. The molecular volume of the head-group and composition of the fatty acyl chains (i.e. saturated vs unsaturated) greatly convey steric features that shape each lipid species. For example, most glycerophospholipids have cylindrical shapes, while cholesterol appears more like a planar molecule with a minimal head-group, sphingomyelin shows more a truncated inverted-cone shape, and most glycosphingolipids (galactosylceramides, sulfatides) and their lysosphingolipid versions (psychosine, sulfopsychosine) are inverted cones where the ceramide/sphingoid tail is topped with outward large sugar heads (Fig. 6A). Thus, the type and abundance of a particular lipid significantly influence the architecture of the raft, and consequently, modify membrane behavior (McMahon and Boucrot, 2015), including bending (Cooke and Deserno, 2006), surface tension and force sensing (Mollinedo and Gajate, 2015) and cell signalling (Gomez-Mouton et al., 2004; Iwabuchi et al., 2010).
The formation of lipid rafts is greatly determined by the melting temperature of the lipids. For example, glycerophospholipids have low-melting temperature while glycosphingolipids and cholesterol have higher-melting temperature (de Almeida et al., 2003). Hence, at physiological temperature, cholesterol and sphingolipids tend to coalesce in more rigid domains that “raft” within the more fluid non-raft areas of the cell membrane composed by low-melting point glycerophospholipids (Bagatolli et al., 2010). Therefore, one can intuitively and easily understand that the lipid composition of the membrane as a whole and of lipid rafts in particular is crucial in the formation, maintenance and dynamics of raft behavior. Evidence for the existence of lipid rafts (Ramstedt and Slotte, 2002) has been provided by multiple studies using planar lipid systems (Fidorra et al., 2006), giant unilamellar vesicles (Dietrich et al., 2001; Kahya et al., 2003; Pinto et al., 2008) and cell-derived PM (Baumgart et al., 2007; Bernardino de la Serna et al., 2004; Plasencia et al., 2007).
The physicochemical characteristics of lipid rafts enable to envision local semi-rigid membrane areas where signalling pathways operate at optimal rates (Fig. 6A). For example, transduction of the IGF signal involves binding of IGF to the IGF-receptor, and activation of a complex cascade of events, involving multiple steps occurring at the membrane such as production of PIP3 via PI3K, which is needed for the subsequent phosphorylation of AKT (Sural-Fehr et al., 2019). Similarly, other signalling pathways key for neural cells such as those mediated by PDGFRα, transferrin receptor, EGF receptor and Notch receptor are also associated with lipid rafts. Hence, lipid rafts act as true “signalosomes” with the capacity to regulate multiple vital signalling pathways (Marin et al., 2013).
Thus, because of their architecture, it is easy to understand how even small changes in lipid raft components may exert large detrimental effects on cells through deregulation of specific raft-associated signalling pathways. In fact, in most lipidoses, alteration of lipid homeostasis leads to changes in the physiological and structural architecture of the membrane and lipid rafts. Altering lipid composition highly impacts on membrane fluidity and curvature, two properties that influence successful interactions between the cell and its environment. Fluidity reflects the average membrane viscosity generated by the rotational and lateral mobility of individual molecules and their interactions with surrounding molecules (Lenaz, 1987). Fluidity is a key property that greatly modifies positioning of proteins and lipids within the membrane. For example, short and unsaturated lipids tend to increase fluid membranes while long and saturated fatty acid chains of sphingolipids as well as cholesterol tend to decrease fluidity. Expectedly, gangliosides (Nishio et al., 2004), sphingomyelin (Galvan et al., 2008; Koike et al., 1998; von Einem et al., 2012), sulfatides (Givogri & Bongarzone, unpublished), and psychosine (D’Auria et al., 2017; Hawkins-Salsbury et al., 2013; White et al., 2011; White et al., 2009) seem to decrease fluidity leading to abnormal spatial distribution of lipid raft components and limiting the proper mobility of raft-proteins and lipids (Fig. 6B). This effect may negatively regulate the interaction of partners of a given signalling pathway. For example, sulfatides are preferentially segregated to detergent resistant membranes or lipid rafts (Moyano et al., 2014). In MLD, the sharp increase of raft sulfatides causes dysregulation of the tyrosine receptor PDGFRα (Pituch et al., 2015), interfering with proper activation of downstream AKT after stimulation of with PDGF, (Pituch et al., 2015). Likewise, the accumulation of psychosine in lipid rafts of Krabbe’s disease alters multiple signalling pathways including PKC (Hawkins-Salsbury et al., 2013; White et al., 2011; White et al., 2009), IGF-receptor (Sural-Fehr et al., 2019), phosphatases (Cantuti Castelvetri et al., 2013; Cantuti-Castelvetri and Bongarzone, 2016; Cantuti-Castelvetri et al., 2012; Castelvetri et al., 2011) and Akt (Cantuti-Castelvetri et al., 2015). As we see, the abnormal accumulation of lipid species in a given lipidosis may elicit multiple secondary cascades of pathogenic mechanisms by affecting membrane fluidity and decreasing signal transduction efficacy. The consequence of this has the potential to affect multiple cellular responses such as remyelination, inflammation, neurodegeneration, synaptic activity and cognition.
In addition to fluidity, membrane curvature is crucial to regulate membrane processes, such as cell shape, vesicular secretion or synaptic activity. Not surprisingly, lipid shapes have a significant effect on membrane bending. Changes in membrane curvature may have a direct contribution to some of the morphological alterations observed in sphingolipidoses by promoting the physical loss of key signalling components (Fig. 5C). For example, accumulation of psychosine not only affects the composition of lipid rafts (White et al., 2009) but also promotes the vesiculation or shedding of membranes (D’Auria et al., 2017). This exacerbated membrane shedding may be significant to understand axonal swelling (Castelvetri et al., 2011) and demyelination in KD and other lipodoses. For example, neurons from GM1 and GM2 gangliosidoses also show large axonal and dendritic swellings (Purpura, 1978; Purpura and Baker, 1977), which could be directly caused by abnormal vesiculation of the membrane.
In conclusion, lipid rafts are crucial to the structure, shape, and function of the cell membrane and pathological changes of their components such as those seen in several lipidoses may have dire consequences including abnormal rigidity, excessive bending, swelling and vesiculation and triggering of pathogenic responses.
9. Final remarks
Since the first description of LSDs, there has been a remarkable effort to understand their pathophysiology. The generation of animal models that recapitulate human disease was key to achieve this goal. Although new cellular and signalling pathways have been linked to dysfunctional neural cells in these pathologies, there is still much to be discovered on how deregulation on the homeostasis of sphingolipids and cholesterol affect the nervous system and specifically neurons and glial cells. New animal models incorporating human mutations and the use of human iPS cells from affected patients will be important paradigms to study pathogenic mechanisms and to test treatments. Importantly, models that resemble adult forms of LSD are largely missing and will be greatly needed to reveal new cellular pathogenic mechanisms underpinning late onset neurodegeneration.
Acknowledgments
This work was supported with funding from the National Institutes of Health (RO1 NS065808-08) and The Legacy of Angels Foundation to ERB; National Center for Advancing Translational Sciences, National Institutes of Health to SG (UL1TR002003); Department of Chemistry, College of Liberal Arts and Sciences, UIC, and the Together Strong-NPC Foundation to SMC.
References
- Abdelkarim H, et al. , 2018. Alpha-Synuclein interacts directly but reversibly with psychosine: implications for alpha-synucleinopathies. Sci. Rep 8, 12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida RF, et al. , 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J 85, 2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphonse PA, Jones PJ, 2016. Revisiting human cholesterol synthesis and absorption: the reciprocity paradigm and its key regulators. Lipids. 51, 519–536. [DOI] [PubMed] [Google Scholar]
- Amick J, et al. , 2016. C9orf72 binds SMCR8, localizes to lysosomes, and regulates mTORC1 signaling. Mol. Biol. Cell 27, 3040–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amick J, et al. , 2020. PQLC2 recruits the C9orf72 complex to lysosomes in response to cationic amino acid starvation. J. Cell Biol 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo MEG, et al. , 2020. Lysosomal size matters. Traffic. 21, 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas F, et al. , 2017. Intracellular cholesterol trafficking and impact in neurodegeneration. Front. Mol. Neurosci 10, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad O, et al. , 2017. Altered differentiation potential of Gaucher’s disease iPSC neuronal progenitors due to Wnt/beta-catenin Downregulation. Stem Cell Rep. 9, 1853–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagatolli LA, et al. , 2010. An outlook on organization of lipids in membranes: searching for a realistic connection with the organization of biological membranes. Prog. Lipid Res 49, 378–389. [DOI] [PubMed] [Google Scholar]
- Bagel JH, et al. , 2013. Electrodiagnostic testing and histopathologic changes confirm peripheral nervous system myelin abnormalities in the feline model of Niemann-pick disease type C. J. Neuropathol. Exp. Neurol 72, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki L, et al. , 2008. Wild-type but not FAD mutant presenilin-1 prevents neuronal degeneration by promoting phosphatidylinositol 3-kinase neuroprotective signaling. J. Neurosci 28, 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabio A, Bonifacino JS, 2020. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol 21, 101–118. [DOI] [PubMed] [Google Scholar]
- Ballabio A, Gieselmann V, 2009. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta 1793, 684–696. [DOI] [PubMed] [Google Scholar]
- Balu D, et al. , 2019. The role of APOE in transgenic mouse models of AD. Neurosci. Lett 707, 134285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, et al. , 1999. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J. Neurosci 19, 7913–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron W, et al. , 2014. Sulfatide-mediated control of extracellular matrix-dependent oligodendrocyte maturation. Glia. 62, 927–942. [DOI] [PubMed] [Google Scholar]
- Bartolomeo R, et al. , 2017. mTORC1 hyperactivation arrests bone growth in lysosomal storage disorders by suppressing autophagy. J. Clin. Invest 127, 3717–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi MT, et al. , 2000. Cloning of the gene encoding a novel integral membrane protein, mucolipidin-and identification of the two major founder mutations causing mucolipidosis type IV. Am. J. Hum. Genet 67, 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart T, et al. , 2007. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U. S. A 104, 3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet AL, et al. , 1980. Acidic phospholipases in cultured human fibroblasts: deficiency of phospholipase C in Niemann-Pick disease. Clin. Chim. Acta 108, 403–414. [DOI] [PubMed] [Google Scholar]
- Bergner CG, et al. , 2019. Microglia damage precedes major myelin breakdown in X-linked adrenoleukodystrophy and metachromatic leukodystrophy. Glia. 67, 1196–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino de la Serna J, et al. , 2004. Cholesterol rules: direct observation of the coexistence of two fluid phases in native pulmonary surfactant membranes at physiological temperatures. J. Biol. Chem 279, 40715–40722. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, et al. , 2018. Long-term treatment of Niemann-Pick type C1 disease with Intrathecal 2-Hydroxypropyl-beta-Cyclodextrin. Pediatr. Neurol 80, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, et al. , 2005. Deregulation of the phosphatidylinositol-3 kinase signaling cascade is associated with neurodegeneration in Npc1−/− mouse brain. Am. J. Pathol 167, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi A, et al. , 2013. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 341, 1233158. [DOI] [PubMed] [Google Scholar]
- Blomqvist M, et al. , 2009. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur. J. Immunol 39, 1726–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Neefjes J, 2017. Moving and positioning the endolysosomal system. Curr. Opin. Cell Biol 47, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher AA, et al. , 2015. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: the largest single-institution cohort report. Orphanet. J. Rare. Dis 10, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury A, et al. , 2016. Cerebrospinal fluid Calbindin D concentration as a biomarker of cerebellar disease progression in Niemann-Pick type C1 disease. J. Pharmacol. Exp. Ther 358, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury AM, et al. , 2018. AAVrh10 gene therapy ameliorates central and peripheral nervous system disease in canine globoid cell leukodystrophy (Krabbe disease). Hum. Gene Ther 29, 785–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan JW, et al. , 2009. The integral membrane of lysosomes: its proteins and their roles in disease. J. Proteome 72, 23–33. [DOI] [PubMed] [Google Scholar]
- Canterini S, et al. , 2017. Shortened primary cilium length and dysregulated Sonic hedgehog signaling in Niemann-Pick C1 disease. Hum. Mol. Genet 26, 2277–2289. [DOI] [PubMed] [Google Scholar]
- Cantuti Castelvetri L, et al. , 2013. The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. J. Neurosci 33, 10048–10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Bongarzone ER, 2016. Synaptic failure: the achilles tendon of sphingolipidoses. J. Neurosci. Res 94, 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, et al. , 2012. Psychosine induces the dephosphorylation of neurofilaments by deregulation of PP1 and PP2A phosphatases. Neurobiol. Dis 46, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, et al. , 2015. Mechanism of neuromuscular dysfunction in Krabbe disease. J. Neurosci 35, 1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carquin M, et al. , 2016. Recent progress on lipid lateral heterogeneity in plasma membranes: from rafts to submicrometric domains. Prog. Lipid Res 62, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Aubourg P, 2008. Hematopoietic stem cell gene therapy in hurler syndrome, globoid cell leukodystrophy, metachromatic leukodystrophy and X-adrenoleukodystrophy. Curr. Opin. Mol. Ther 10, 471–478. [PubMed] [Google Scholar]
- Castellano BM, et al. , 2017. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science. 355, 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelvetri LC, et al. , 2011. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 122, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler RJ, et al. , 2017. Systemic AAV9 gene therapy improves the lifespan of mice with Niemann-Pick disease, type C1. Hum. Mol. Genet 26, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, et al. , 2017. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet 49, 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew H, et al. , 2020. Involvement of lipids in Alzheimer’s disease pathology and potential therapies. Front. Physiol 11, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, et al. , 1997. Tissue culture model of Krabbe’s disease: psychosine cytotoxicity in rat oligodendrocyte culture. Dev. Neurosci 19, 321–327. [DOI] [PubMed] [Google Scholar]
- Chu BB, et al. , 2015. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell. 161, 291–306. [DOI] [PubMed] [Google Scholar]
- Claycomb KI, et al. , 2014. Aberrant production of tenascin-C in globoid cell leukodystrophy alters psychosine-induced microglial functions. J. Neuropathol. Exp. Neurol 73, 964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cologna SM, Rosenhouse-Dantsker A, 2019. Insights into the molecular mechanisms of cholesterol binding to the NPC1 and NPC2 proteins. Adv. Exp. Med. Biol 1135, 139–160. [DOI] [PubMed] [Google Scholar]
- Cologna SM, et al. , 2012. Quantitative proteomic analysis of Niemann-Pick disease, type C1 cerebellum identifies protein biomarkers and provides pathological insight. PLoS One 7, e47845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cologna SM, et al. , 2014. Human and mouse neuroinflammation markers in Niemann-Pick disease, type C1. J. Inherit. Metab. Dis 37, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conus S, Simon HU, 2008. Cathepsins: key modulators of cell death and inflammatory responses. Biochem. Pharmacol 76, 1374–1382. [DOI] [PubMed] [Google Scholar]
- Cooke IR, Deserno M, 2006. Coupling between lipid shape and membrane curvature. Biophys. J 91, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougnoux A, et al. , 2018. Microglia activation in Niemann-Pick disease, type C1 is amendable to therapeutic intervention. Hum. Mol. Genet 27, 2076–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria L, et al. , 2017. Psychosine enhances the shedding of membrane microvesicles: implications in demyelination in Krabbe’s disease. PLoS One 12, e0178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CD, et al. , 2009. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One 4, e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies-Thompson J, et al. , 2016. Reduced myelin water in the White matter tracts of patients with Niemann-Pick disease type C. AJNR Am. J. Neuroradiol 37, 1487–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caprio J, et al. , 1992. Bile acid and sterol solubilization in 2-hydroxypropyl-beta-cyclodextrin. J. Lipid Res 33, 441–443. [PubMed] [Google Scholar]
- De Duve C, et al. , 1955. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J 60, 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C, et al. , 2001. Lipid rafts reconstituted in model membranes. Biophys. J 80, 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, 2017. Lipid involvement in neurodegenerative diseases of the motor system: insights from Lysosomal storage diseases. Front. Mol. Neurosci 10, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Czaja MJ, 2011. Regulation of lipid droplets by autophagy. Trends Endocrinol. Metab 22, 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R, et al. , 2016. Endosome-ER contacts control actin nucleation and Retromer function through VAP-dependent regulation of PI4P. Cell. 166, 408–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, et al. , 2010. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat. Commun 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen LW, et al. , 1980. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 103, 695–710. [DOI] [PubMed] [Google Scholar]
- de Duve C, 2005. The lysosome turns fifty. Nat. Cell Biol 7, 847–849. [DOI] [PubMed] [Google Scholar]
- Düvel K, et al. , 2010. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183. 10.1016/j.molcel.2010.06.022. PMID: 20670887; PMCID: PMC2946786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M, et al. , 2007. Sulfatide storage in neurons causes hyperexcitability and axonal degeneration in a mouse model of metachromatic leukodystrophy. J. Neurosci 27, 9009–9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthymiou AG, et al. , 2015. Rescue of an in vitro neuron phenotype identified in Niemann-Pick disease, type C1 induced pluripotent stem cell-derived neurons by modulating the WNT pathway and calcium signaling. Stem Cells Transl. Med 4, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Einem B, et al. , 2012. Cholesterol-dependent energy transfer between fluorescent proteins-insights into protein proximity of APP and BACE1 in different membranes in Niemann-pick type C disease cells. Int. J. Mol. Sci 13, 15801–15812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar ML, et al. , 2005. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N. Engl. J. Med 352, 2069–2081. [DOI] [PubMed] [Google Scholar]
- Eskelinen EL, et al. , 2004. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol. Biol. Cell 15, 3132–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabelo N, et al. , 2011. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol. Med 17, 1107–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, et al. , 2013. Identification of Niemann-Pick C1 disease biomarkers through sphingolipid profiling. J. Lipid Res 54, 2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltes M, et al. , 2020. Monitoring the itinerary of lysosomal cholesterol in Niemann-Pick type C1-deficient cells after cyclodextrin treatment. J. Lipid Res 61, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, et al. , 2019. Stimulation of mGluR1/5 improves defective internalization of AMPA receptors in NPC1 mutant mouse. Mar 14 Cereb Cortex 30, 1465–1480. 10.1093/cercor/bhz179. PMID: 31599924. [DOI] [PubMed] [Google Scholar]
- Fidorra M, et al. , 2006. Absence of fluid-ordered/fluid-disordered phase coexistence in ceramide/POPC mixtures containing cholesterol. Biophys. J 90, 4437–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, et al. , 2010. ABC transporters, atherosclerosis and inflammation. Atherosclerosis. 211, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fog CK, Kirkegaard T, 2019. Animal models for Niemann-Pick type C: implications for drug discovery & development. Expert Opin. Drug Discovery 14, 499–509. [DOI] [PubMed] [Google Scholar]
- Formichi P, et al. , 2018. Primary cilium alterations and expression changes of Patched1 proteins in Niemann-Pick type C disease. J. Cell. Physiol 233, 663–672. [DOI] [PubMed] [Google Scholar]
- Frech MJ, et al. , 2015. Cyclodextrin alters GABAergic input to CA1 pyramidal cells in wild-type but not in NPC1-deficient mice. Biores Open Access. 4, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu R, et al. , 2010. Oxidative stress in Niemann-Pick disease, type C. Mol. Genet. Metab 101, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, et al. , 2009. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J. Neurosci. Res 87, 1748–1759. [DOI] [PubMed] [Google Scholar]
- Galvan C, et al. , 2008. Anomalous surface distribution of glycosyl phosphatidyl inositol- anchored proteins in neurons lacking acid sphingomyelinase. Mol. Biol. Cell 19, 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Robles AA, et al. , 2016. Use of 2 hydroxypropyl-beta-cyclodextrin therapy in two adult Niemann pick type C patients. J. Neurol. Sci 366, 65–67. [DOI] [PubMed] [Google Scholar]
- Gelsthorpe ME, et al. , 2008. Niemann-Pick type C1 I1061T mutant encodes a functional protein that is selected for endoplasmic reticulum-associated degradation due to protein misfolding. J. Biol. Chem 283, 8229–8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, et al. , 2002. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 109, 437–450. [DOI] [PubMed] [Google Scholar]
- Ghanevati M, Miller CA, 2005. Phospho-beta-catenin accumulation in Alzheimer’s disease and in aggresomes attributable to proteasome dysfunction. J. Mol. Neurosci 25, 79–94. [DOI] [PubMed] [Google Scholar]
- Giese AK, et al. , 2015. A novel, highly sensitive and specific biomarker for Niemann-Pick type C1 disease. Orphanet. J. Rare. Dis 10, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieselmann V, 2008. Metachromatic leukodystrophy: genetics, pathogenesis and therapeutic options. Acta Paediatr. 97, 15–21. [DOI] [PubMed] [Google Scholar]
- Gieselmann V, et al. , 1998. Metachromatic leukodystrophy: molecular genetics and an animal model. J. Inherit. Metab. Dis 21, 564–574. [DOI] [PubMed] [Google Scholar]
- Giri S, et al. , 2006. Krabbe disease: psychosine-mediated activation of phospholipase A2 in oligodendrocyte cell death. J. Lipid Res 47, 1478–1492. [DOI] [PubMed] [Google Scholar]
- Giri S, et al. , 2008. The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: implication for Krabbe disease. J. Neurochem 105, 1820–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mouton C, et al. , 2004. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J. Cell Biol 164, 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar S, Ferguson SM, 2016. Lysosomes relax in the cellular suburbs. J. Cell Biol 212, 617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar S, et al. , 2015. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc. Natl. Acad. Sci. U. S. A 112, E3699–E3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano AC, et al. , 2016. Krabbe disease: involvement of connexin43 in the apoptotic effects of sphingolipid psychosine on mouse oligodendrocyte precursors. Apoptosis. 21, 25–35. [DOI] [PubMed] [Google Scholar]
- Groeschel S, et al. , 2012. Cerebral gray and white matter changes and clinical course in metachromatic leukodystrophy. Neurology. 79, 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeschel S, et al. , 2016. Long-term outcome of allogeneic hematopoietic stem cell transplantation in patients with juvenile metachromatic Leukodystrophy compared with nontransplanted control patients. JAMA Neurol. 73, 1133–1140. [DOI] [PubMed] [Google Scholar]
- Guillen-Samander A, et al. , 2019. PDZD8 mediates a Rab7-dependent interaction of the ER with late endosomes and lysosomes. Proc. Natl. Acad. Sci. U. S. A 116, 22619–22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, et al. , 2016. Niemann-Pick type C2 deficiency impairs autophagy-lysosomal activity, mitochondrial function, and TLR signaling in adipocytes. J. Lipid Res 57, 1644–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson KH, Hagberg B, 1971. The incidence and genetics of metachromatic leucodystrophy in northern Sweden. Acta Paediatr. Scand 60, 585–590. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, et al. , 2019. Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease. J. Neuroinflammation 16, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer I, et al. , 2012. Lipids and lysosomes. Curr. Drug Metab. 13, 1371–1387. [DOI] [PubMed] [Google Scholar]
- Haq E, et al. , 2003. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. J. Neurochem 86, 1428–1440. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, 2010. Sphingolipids in neurodegeneration. NeuroMolecular Med. 12, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins-Salsbury JA, et al. , 2013. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J. Lipid Res 54, 3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, et al. , 2014. Deficiency of patched 1-induced Gli1 signal transduction results in astrogenesis in Swedish mutated APP transgenic mice. Hum. Mol. Genet 23, 6512–6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B, et al. , 1996. Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc. Natl. Acad. Sci. U. S. A 93, 14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara Y, et al. , 2004. Sulfatide is a negative regulator of oligodendrocyte differentiation: development in sulfatide null mice. Glia. 45, 269–277. [DOI] [PubMed] [Google Scholar]
- Hirahara Y, et al. , 2017. Sulfatide species with various fatty acid chains in oligodendrocytes at different developmental stages determined by imaging mass spectrometry. J. Neurochem 140, 435–450. [DOI] [PubMed] [Google Scholar]
- Hoglinger D, et al. , 2015. Intracellular sphingosine releases calcium from lysosomes. Elife. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger D, et al. , 2019. NPC1 regulates ER contacts with endocytic organelles to mediate cholesterol egress. Nat. Commun 10, 4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honke K, 2013. Biosynthesis and biological function of sulfoglycolipids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 89, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, et al. , 2018. The role of fluoxetine in activating Wnt/beta-catenin signaling and repressing beta-amyloid production in an Alzheimer mouse model. Front. Aging Neurosci 10, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mahley RW, 2014. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol. Dis 72 Pt A, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MP, et al. , 2018. AAV9 intracerebroventricular gene therapy improves lifespan, locomotor function and pathology in a mouse model of Niemann-Pick type C1 disease. Hum. Mol. Genet 27, 3079–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius MJ, et al. , 1986. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc. Natl. Acad. Sci. U. S. A 83, 1125–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi K, et al. , 2013. MMP-3 mediates psychosine-induced globoid cell formation: implications for leukodystrophy pathology. Glia. 61, 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, et al. , 2018. Developmental defects and aberrant accumulation of endogenous psychosine in oligodendrocytes in a murine model of Krabbe disease. Neurobiol. Dis 120, 51–62. [DOI] [PubMed] [Google Scholar]
- Inui K, et al. , 1988. Metabolism of cerebroside sulfate and subcellular distribution of its metabolites in cultured skin fibroblasts from controls, metachromatic leukodystrophy, and globoid cell leukodystrophy. J. Clin. Invest 81, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac G, et al. , 2006. Sulfatide with short fatty acid dominates in astrocytes and neurons. FEBS J. 273, 1782–1790. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, et al. , 2002. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J. Neurosci 22, 6507–6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K, et al. , 2010. Significance of glycosphingolipid fatty acid chain length on membrane microdomain-mediated signal transduction. FEBS Lett. 584, 1642–1652. [DOI] [PubMed] [Google Scholar]
- Jahng A, et al. , 2004. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med 199, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaishy B, Abel ED, 2016. Lipids, lysosomes, and autophagy. J. Lipid Res 57, 1619–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SB, et al. , 2008. Sulfatide, a major lipid component of myelin sheath, activates inflammatory responses as an endogenous stimulator in brain-resident immune cells. J. Immunol 181, 8077–8087. [DOI] [PubMed] [Google Scholar]
- Jiang X, et al. , 2011. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J. Lipid Res 52, 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, et al. , 2016. Development of a bile acid-based newborn screen for Niemann-Pick disease type C. Sci. Transl. Med 8, 337ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, et al. , 2017. Sodium selenate activated Wnt/beta-catenin signaling and repressed amyloid-beta formation in a triple transgenic mouse model of Alzheimer’s disease. Exp. Neurol 297, 36–49. [DOI] [PubMed] [Google Scholar]
- Jin U, et al. , 2019. Cholesterol metabolism in the brain and its association with Parkinson’s disease. Exp. Neurobiol 28, 554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, et al. , 2016. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol 212, 677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahya N, et al. , 2003. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem 278, 28109–28115. [DOI] [PubMed] [Google Scholar]
- Karumuthil-Melethil S, et al. , 2016. Intrathecal administration of AAV/GALC vectors in 10-11-day-old twitcher mice improves survival and is enhanced by bone marrow transplant. J. Neurosci. Res 94, 1138–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BE, et al. , 2014. Adaptations of energy metabolism associated with increased levels of mitochondrial cholesterol in Niemann-Pick type C1-deficient cells. J. Biol. Chem 289, 16278–16289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn KJ, et al. , 2016. A Drosophila model of Neuronopathic Gaucher disease demonstrates Lysosomal-Autophagic defects and altered mTOR signalling and is functionally rescued by Rapamycin. J. Neurosci 36, 11654–11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriyama Y, Nochi H, 2015. The function of autophagy in neurodegenerative diseases. Int. J. Mol. Sci 16, 26797–26812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, et al. , 2011. Oxidative stress activates the c-Abl/p73 proapoptotic pathway in Niemann-Pick type C neurons. Neurobiol. Dis 41, 209–218. [DOI] [PubMed] [Google Scholar]
- Koike T, et al. , 1998. Decreased membrane fluidity and unsaturated fatty acids in Niemann-pick disease type C fibroblasts. Biochim. Biophys. Acta 1406, 327–335. [DOI] [PubMed] [Google Scholar]
- Kolodny EH, Cable WJ, 1982. Inborn errors of metabolism. Ann. Neurol 11, 221–232. [DOI] [PubMed] [Google Scholar]
- Kolodny EH, Fluharty AL, 1995. Metachromatic leukodystrophy and multiple sulfatase defi-ciency: sulfatide lipidosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D (Eds.), The Metabolic and Molecular Bases of Inherited Disease, 7th edn. McGraw-Hill, New York, pp. 2693–2740. [Google Scholar]
- Kondo Y, et al. , 2011. Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy. J. Neurosci 31, 3610–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, et al. , 2011. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol 13, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe K, 1916. A new familial, infantile form of diffuse brain. Sclerosis. Brain 39, 74–114. [DOI] [PubMed] [Google Scholar]
- Krivit W, et al. , 1998. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N. Engl. J. Med 338, 1119–1126. [DOI] [PubMed] [Google Scholar]
- Kumar N, et al. , 2018. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol 217, 3625–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, et al. , 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 137, 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann RH, 2003. Miglustat. Oxford GlycoSciences/Actelion. Curr. Opin. Investig. Drugs 4, 472–479. [PubMed] [Google Scholar]
- Lachmann RH, et al. , 2004. Treatment with miglustat reverses the lipid-trafficking defect in Niemann-Pick disease type C. Neurobiol. Dis. 16, 654–658. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Bar-Peled L, 2019. Lysosome: the metabolic signaling hub. Traffic. 20, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Y, et al. , 1997. The fate of cholesterol exiting lysosomes. J. Biol. Chem 272, 17018–17022. [DOI] [PubMed] [Google Scholar]
- Lawrence RE, Zoncu R, 2019. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol 21, 133–142. [DOI] [PubMed] [Google Scholar]
- Lee JH, et al. , 2015. Presenilin 1 maintains Lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 12, 1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G, 1987. Lipid fluidity and membrane protein dynamics. Biosci. Rep 7, 823–837. [DOI] [PubMed] [Google Scholar]
- Leung K, et al. , 2019. A DNA nanomachine chemically resolves lysosomes in live cells. Nat. Nanotechnol 14, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine SM, Brown DC, 1997. IL-6 and TNFalpha expression in brains of twitcher, quaking and normal mice. J. Neuroimmunol 73, 47–56. [DOI] [PubMed] [Google Scholar]
- Li J, Pfeffer SR, 2016. Lysosomal membrane glycoproteins bind cholesterol and contribute to lysosomal cholesterol export. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, et al. , 2005. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J. Cell Biol 169, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. , 2017. 3.3 A structure of Niemann-Pick C1 protein reveals insights into the function of the C-terminal luminal domain in cholesterol transport. Proc. Natl. Acad. Sci. U. S. A 114, 9116–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YC, et al. , 2019. RNA granules hitchhike on lysosomes for long-distance transport, using Annexin A11 as a molecular tether. Cell 179, 147–164.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CY, et al. , 2019. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nat. Cell Biol 21, 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K, 2010. Lipid rafts as a membrane-organizing principle. Science. 327, 46–50. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, et al. , 2008. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med 14, 1247–1255. [DOI] [PubMed] [Google Scholar]
- Lubke T, et al. , 2009. Proteomics of the lysosome. Biochim. Biophys. Acta 1793, 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi P, et al. , 2005. Biochemical and pathological evaluation of long-lived mice with globoid cell leukodystrophy after bone marrow transplantation. Mol. Genet. Metab 86, 150–159. [DOI] [PubMed] [Google Scholar]
- Maarup TJ, et al. , 2015. Intrathecal 2-hydroxypropyl-beta-cyclodextrin in a single patient with Niemann-Pick C1. Mol. Genet. Metab 116, 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik BR, et al. , 2019. Autophagic and endo-lysosomal dysfunction in neurodegenerative disease. Mol. Brain 12, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbois BN, et al. , 2000. Analysis of sulfatide from rat cerebellum and multiple sclerosis white matter by negative ion electrospray mass spectrometry. Biochim. Biophys. Acta 1484, 59–70. [DOI] [PubMed] [Google Scholar]
- Maricic I, et al. , 2014. Recognition of lysophosphatidylcholine by type II NKT cells and protection from an inflammatory liver disease. J. Immunol 193, 4580–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R, et al. , 2013. Lipid raft disarrangement as a result of neuropathological progresses: a novel strategy for early diagnosis? Aug 15 Neuroscience 245, 26–39. 10.1016/j.neuroscience.2013.04.025. Epub 2013 Apr 22. PMID: 23618758. [DOI] [PubMed] [Google Scholar]
- Marshall MS, et al. , 2018a. Long-term improvement of neurological signs and metabolic dysfunction in a mouse model of Krabbe’s disease after global gene therapy. Mol. Ther 26, 874–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MS, et al. , 2018b. Analysis of age-related changes in psychosine metabolism in the human brain. PLoS One 13, e0193438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews ES, Appel B, 2016. Cholesterol biosynthesis supports myelin gene expression and axon Ensheathment through modulation of P13K/Akt/mTor signaling. J. Neurosci 36, 7628–7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzacuva F, et al. , 2016. Identification of novel bile acids as biomarkers for the early diagnosis of Niemann-Pick C disease. FEBS Lett. 590, 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E, 2015. Membrane curvature at a glance. J. Cell Sci 128, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, et al. , 2011. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N, et al. , 2014. Long-term correction of biochemical and neurological abnormalities in MLD mice model by neonatal systemic injection of an AAV serotype 9 vector. Gene Ther. 21, 427–433. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C, 2015. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul 57, 130–146. [DOI] [PubMed] [Google Scholar]
- Moreau K, et al. , 2013. Connections between SNAREs and autophagy. Trends Biochem. Sci 38, 57–63. [DOI] [PubMed] [Google Scholar]
- Moyano AL, et al. , 2013. Levels of plasma sulfatides C18: 0 and C24: 1 correlate with disease status in relapsing-remitting multiple sclerosis. J. Neurochem 127, 600–604. [DOI] [PubMed] [Google Scholar]
- Moyano AL, et al. , 2014. Distribution of C16:0, C18:0, C24:1, and C24:0 sulfatides in central nervous system lipid rafts by quantitative ultra-high-pressure liquid chromatography tandem mass spectrometry. Dec 15 Anal Biochem. 467, 31–39. 10.1016/j.ab.2014.08.033. Epub 2014 Sep 7. PMID: 25205652. [DOI] [PubMed] [Google Scholar]
- Moyano AL, et al. , 2016. Sulfatides in extracellular vesicles isolated from plasma of multiple sclerosis patients. J. Neurosci. Res 94, 1579–1587. [DOI] [PubMed] [Google Scholar]
- Napolitano G, Ballabio A, 2016. TFEB at a glance. J. Cell Sci 129, 2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano G, et al. , 2018. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat. Commun 9, 3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EB, et al. , 1996. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J. Biol. Chem 271, 21604–21613. [DOI] [PubMed] [Google Scholar]
- Newton J, et al. , 2018. Niemann-pick type C disease: the atypical sphingolipidosis. Adv. Biol. Regul 70, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, et al. , 1999. Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of beta-catenin, a component of the presenilin protein complex. Nat. Med 5, 164–169. [DOI] [PubMed] [Google Scholar]
- Nishio M, et al. , 2004. Overexpressed GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells. J. Biol. Chem 279, 33368–33378. [DOI] [PubMed] [Google Scholar]
- Nixon RA, 2020. The aging lysosome: an essential catalyst for late-onset neurodegenerative diseases. Biochim. Biophys. Acta, Proteins Proteomics 1868, 140443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Yang DS, 2011. Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol. Dis 43, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT, Cammer W, 1984. Isolation and characterization of myelin. In: Morell P (Ed.), Myelin, 2nd ed. Plenum, New York, pp. 147–180. [Google Scholar]
- Novikoff AB, et al. , 1956. Electron microscopy of lysosomerich fractions from rat liver. J. Biophys. Biochem. Cytol 2, 179–184. [PMC free article] [PubMed] [Google Scholar]
- Ohno M, et al. , 1993. Proliferation of microglia/macrophages in the demyelinating CNS and PNS of twitcher mouse. Brain Res. 602, 268–274. [DOI] [PubMed] [Google Scholar]
- Ory DS, et al. , 2017. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-pick disease, type C1: a non-randomised, open-label, phase 1-2 trial. Lancet. 390, 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco CD, et al. , 2007. Autophagy in Niemann-pick C disease is dependent upon Beclin-1 and responsive to lipid trafficking defects. Hum. Mol. Genet 16, 1495–1503. [DOI] [PubMed] [Google Scholar]
- Palmieri M, et al. , 2011. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet 20, 3852–3866. [DOI] [PubMed] [Google Scholar]
- Papandreou A, Gissen P, 2016. Diagnostic workup and management of patients with suspected Niemann-pick type C disease. Ther. Adv. Neurol. Disord 9, 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti G, et al. , 2013. New strategies for the treatment of lysosomal storage diseases (review). Int. J. Mol. Med 31, 11–20. [DOI] [PubMed] [Google Scholar]
- Patterson MC, et al. , 2007. Miglustat for treatment of Niemann-pick C disease: a randomised controlled study. Lancet Neurol. 6, 765–772. [DOI] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, 2016. The lysosome as a regulatory hub. Annu. Rev. Cell Dev. Biol 32, 223–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergande MR, et al. , 2019a. Differential proteomics reveals miR-155 as a novel Indicator of liver and spleen pathology in the symptomatic Niemann-pick disease, type C1 mouse model. Molecules. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergande MR, et al. , 2019b. Lipidomic analysis reveals altered fatty acid metabolism in the liver of the symptomatic Niemann-pick, Type C1 Mouse Model. Proteomics 19, e1800285. [DOI] [PubMed] [Google Scholar]
- Pergande MR, et al. , 2019c. Standard-flow LC and thermal focusing ESI elucidates altered liver proteins in late stage Niemann-pick, type C1 disease. Bioanalysis. 11, 1067–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit CS, et al. , 2013. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of rag GTPases. J. Cell Biol 202, 1107–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, 2019. NPC intracellular cholesterol transporter 1 (NPC1)-mediated cholesterol export from lysosomes. J. Biol. Chem 294, 1706–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, Voeltz GK, 2016. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol 17, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet F, et al. , 2012. Correction of brain oligodendrocytes by AAVrh.10 intracerebral gene therapy in metachromatic leukodystrophy mice. Hum. Gene Ther 23, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SN, et al. , 2008. Membrane domain formation, interdigitation, and morphological alterations induced by the very long chain asymmetric C24:1 ceramide. Biophys. J 95, 2867–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pituch KC, et al. , 2015. Dysfunction of platelet-derived growth factor receptor alpha (PDGFRalpha) represses the production of oligodendrocytes from arylsulfatase A-deficient multipotential neural precursor cells. J. Biol. Chem 290, 7040–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia I, et al. , 2007. Direct visualization of lipid domains in human skin stratum corneum’s lipid membranes: effect of pH and temperature. Biophys. J 93, 3142–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM, 2014. Sphingolipid lysosomal storage disorders. Nature. 510, 68–75. [DOI] [PubMed] [Google Scholar]
- Platt FM, 2018. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov 17, 133–150. [DOI] [PubMed] [Google Scholar]
- Platt FM, et al. , 2012. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol 199, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM, et al. , 2018. Lysosomal storage diseases. Nat. Rev. Dis. Primers 4, 27. [DOI] [PubMed] [Google Scholar]
- Pols MS, et al. , 2013. hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat. Commun 4, 1361. [DOI] [PubMed] [Google Scholar]
- Porter FD, Herman GE, 2011. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res 52, 6–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praggastis M, et al. , 2015. A murine Niemann-pick C1 I1061T knock-in model recapitulates the pathological features of the most prevalent human disease allele. J. Neurosci 35, 8091–8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralhada Rao R, et al. , 2013. Sphingolipid metabolic pathway: an overview of major roles played in human diseases. J. Lipids 2013, 178910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, et al. , 2020. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol 21, 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP, 1978. Ectopic dendritic growth in mature pyramidal neurones in human ganglioside storage disease. Nature. 276, 520–521. [DOI] [PubMed] [Google Scholar]
- Purpura KP, Baker HJ, 1977. Neurite induction in mature cortical neurones in feline GM1-ganglioside storage disease. Nature. 266, 553–554. [DOI] [PubMed] [Google Scholar]
- Qiao L, et al. , 2018. Altered myelination in the Niemann-pick type C1 mutant mouse. Histol. Histopathol 33, 1311–1321. [DOI] [PubMed] [Google Scholar]
- Qin EY, et al. , 2012. Bone marrow transplantation increases efficacy of central nervous system-directed enzyme replacement therapy in the murine model of globoid cell leukodystrophy. Mol. Genet. Metab 107, 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein M, et al. , 2017. Decreased calcium flux in Niemann-pick type C1 patient-specific iPSC-derived neurons due to higher amount of calcium-impermeable AMPA receptors. Mol. Cell. Neurosci 83, 27–36. [DOI] [PubMed] [Google Scholar]
- Raiborg C, et al. , 2015. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 520, 234–238. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan H, et al. , 2007. Increasing sulfatide synthesis in myelin-forming cells of arylsulfatase A-deficient mice causes demyelination and neurological symptoms reminiscent of human metachromatic leukodystrophy. J. Neurosci 27, 9482–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Saykin AJ, 2013. Pathways to neurodegeneration: mechanistic insights from GWAS in Alzheimer’s disease, Parkinson’s disease, and related disorders. Am. J. Neurodegener Dis 2, 145–175. [PMC free article] [PubMed] [Google Scholar]
- Ramstedt B, Slotte JP, 2002. Membrane properties of sphingomyelins. FEBS Lett. 531, 33–37. [DOI] [PubMed] [Google Scholar]
- Rauniyar N, et al. , 2015. Quantitative proteomics of human fibroblasts with I1061T mutation in Niemann-pick C1 (NPC1) protein provides insights into the disease pathogenesis. Mol. Cell. Proteomics 14, 1734–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, et al. , 2011. Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy. J. Neurosci 31, 9945–9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha N, et al. , 2009. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J. Cell Biol 185, 1209–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roczniak-Ferguson A, et al. , 2012. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal 5, ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues EM, et al. , 2012. Enhanced beta-secretase processing alters APP axonal transport and leads to axonal defects. Hum. Mol. Genet 21, 4587–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JB, et al. , 2016. Gene therapy for metachromatic leukodystrophy. J. Neurosci. Res 94, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundy K, et al. , 1999. Microphthalmic mice display a B cell deficiency similar to that seen for mast and NK cells. J. Immunol 163, 6671–6678. [PubMed] [Google Scholar]
- Rowland AA, et al. , 2014. ER contact sites define the position and timing of endosome fission. Cell. 159, 1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford AC, et al. , 2006. The mammalian phosphatidylinositol 3-phosphate 5- kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J. Cell Sci 119, 3944–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez PJ, et al. , 2013. Disruption in connexin-based communication is associated with intracellular Ca(2)(+) signal alterations in astrocytes from Niemann-pick type C mice. PLoS One 8, e71361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, et al. , 2016. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci 19, 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Klumperman J, 2009. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol 10, 623–635. [DOI] [PubMed] [Google Scholar]
- Sardiello M, et al. , 2009. A gene network regulating lysosomal biogenesis and function. Science. 325, 473–477. [DOI] [PubMed] [Google Scholar]
- Sarkar S, et al. , 2013. Impaired autophagy in the lipid-storage disorder Niemann-pick type C1 disease. Cell Rep. 5, 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR signaling in growth, metabolism, and disease. Cell. 169, 361–371. [DOI] [PubMed] [Google Scholar]
- Schultz ML, et al. , 2018. Coordinate regulation of mutant NPC1 degradation by selective ER autophagy and MARCH6-dependent ERAD. Nat. Commun 9, 3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Ballabio A, 2014. Lysosome: regulator of lipid degradation pathways. Trends Cell Biol. 24, 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, et al. , 2011. TFEB links autophagy to lysosomal biogenesis. Science. 332, 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, et al. , 2012. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, et al. , 2013. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol 15, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammas H, et al. , 2019. Different Niemann-pick C1 genotypes generate protein phenotypes that vary in their intracellular processing, trafficking and localization. Sci. Rep 9, 5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro EG, et al. , 1994. Characteristics of the dementia in late-onset metachromatic leukodystrophy. Neurology. 44, 662–665. [DOI] [PubMed] [Google Scholar]
- Shen D, et al. , 2012. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun 3, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng R, et al. , 2014. Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling. Nat. Commun 5, 4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff SM, et al. , 2009. Adult CST-null mice maintain an increased number of oligodendrocytes. J. Neurosci. Res 87, 3403–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu R, et al. , 2015. A validated LC-MS/MS assay for quantification of 24(S)-hydroxycholesterol in plasma and cerebrospinal fluid. J. Lipid Res 56, 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu R, et al. , 2019. N-acyl-O-phosphocholineserines: structures of a novel class of lipids that are biomarkers for Niemann-pick C1 disease. J. Lipid Res 60, 1410–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E, 1997. Functional rafts in cell membranes. Nature. 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL, 1972. The fluid mosaic model of the structure of cell membranes. Science. 175, 720–731. [DOI] [PubMed] [Google Scholar]
- Singhal A, et al. , 2018. Hydroxypropyl-beta and -gamma cyclodextrins rescue cholesterol accumulation in Niemann-pick C1 mutant cell via lysosome-associated membrane protein 1. Cell Death Dis. 9, 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, et al. , 2011. Peripheral neuropathy in the Twitcher mouse involves the activation of axonal caspase 3. ASN Neuro. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, et al. , 2014. Neuronal inclusions of alpha-synuclein contribute to the pathogenesis of Krabbe disease. J. Pathol 232, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook ER, et al. , 2014. Innate immune activation in the pathogenesis of a murine model of globoid cell leukodystrophy. Am. J. Pathol 184, 382–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling GP, et al. , 2016. Folliculin directs the formation of a Rab34-RILP complex to control the nutrient-dependent dynamic distribution of lysosomes. EMBO Rep. 17, 823–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, et al. , 1989. Oligodendrocytes but not astrocytes express apolipoprotein E after injury of rat optic nerve. Glia. 2, 170–176. [DOI] [PubMed] [Google Scholar]
- Storch J, Cheruku SR, 2005. Cholesterol transport in lysosomes. In: Lysosomes. Springer US, Boston, MA, pp. 100–111. [Google Scholar]
- Strasser B, et al. , 2011. The V-ATPase proteolipid cylinder promotes the lipid-mixing stage of SNARE-dependent fusion of yeast vacuoles. EMBO J. 30, 4126–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, et al. , 2008. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron. 60, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sural-Fehr T, et al. , 2019. Inhibition of the IGF-1-PI3K-Akt-mTORC2 pathway in lipid rafts increases neuronal vulnerability in a genetic lysosomal glycosphingolipidosis. Dis. Model. Mech 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, 2003. Globoid cell leukodystrophy (Krabbe’s disease): update. J. Child Neurol 18, 595–603. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Stallberg-Stenhagen S, 1968. Changes in the fatty acid composition of cerebrosides and sulfatides of human nervous tissue with age. J. Lipid Res 9, 215–225. [PubMed] [Google Scholar]
- Swetha MG, et al. , 2011. Lysosomal membrane protein composition, acidic pH and sterol content are regulated via a light-dependent pathway in metazoan cells. Traffic. 12, 1037–1055. [DOI] [PubMed] [Google Scholar]
- Tai LM, et al. , 2017. EFAD transgenic mice as a human APOE relevant preclinical model of Alzheimer’s disease. J. Lipid Res 58, 1733–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Suzuki T, 2012. Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res 53, 1437–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, et al. , 2012. MRI characterization of paranodal junction failure and related spinal cord changes in mice. PLoS One 7, e52904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen AM, Zoncu R, 2017. Emerging roles for the lysosome in lipid metabolism. Trends Cell Biol. 27, 833–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscione SA, et al. , 2019. Disease-associated mutations in Niemann-pick type C1 alter ER calcium signaling and neuronal plasticity. J. Cell Biol 218, 4141–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SK, et al. , 2015. Ethosuximide induces hippocampal neurogenesis and reverses cognitive deficits in an amyloid-beta toxin-induced Alzheimer rat model via the phosphatidylinositol 3-kinase (PI3K)/Akt/Wnt/beta-catenin pathway. J. Biol. Chem 290, 28540–28558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh MN, et al. , 2020. Last step in the path of LDL cholesterol from lysosome to plasma membrane to ER is governed by phosphatidylserine. Proc. Natl. Acad. Sci. U. S. A 117, 18521–18529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood KW, et al. , 1996. Quantitative analysis of hydrophobic amine inhibition of intracellular cholesterol transport. J. Lipid Res 37, 1556–1568. [PubMed] [Google Scholar]
- Vacca F, et al. , 2019. Cyclodextrin triggers MCOLN1-dependent endo-lysosome secretion in Niemann-pick type C cells. J. Lipid Res 60, 832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanier MT, Millat G, 2003. Niemann-pick disease type C. Clin. Genet 64, 269–281. [DOI] [PubMed] [Google Scholar]
- Vite CH, et al. , 2015. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann-Pick type C1 disease. Sci. Transl. Med 7, 276ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voccoli V, et al. , 2014. Role of extracellular calcium and mitochondrial oxygen species in psychosine-induced oligodendrocyte cell death. Cell Death Dis. 5, e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, et al. , 2017. A voltage-dependent K(+) channel in the lysosome is required for refilling lysosomal Ca(2+) stores. J. Cell Biol 216, 1715–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassif CA, et al. , 2016. High incidence of unrecognized visceral/neurological late-onset Niemann-pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet. Med 18, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP, 1999. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 22, 103–114. [DOI] [PubMed] [Google Scholar]
- Wenger D, et al. , 2019. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, Antonarakis Ballabio (Eds.), The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York. 10.1036/ommbid.178. [DOI] [Google Scholar]
- Wheeler S, Sillence DJ, 2019. Niemann-pick type C disease: cellular pathology and pharmacotherapy. J. Neurochem 153, 674–692. 10.1111/jnc.14895. Epub 2019 Nov 15. PMID: 31608980. [DOI] [PubMed] [Google Scholar]
- White AB, et al. , 2009. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J. Neurosci 29, 6068–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AB, et al. , 2011. Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease. J. Neurosci. Res 89, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm LP, et al. , 2017. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 36, 1412–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler AM, et al. , 2011. The lipid sulfatide is a novel myelin-associated inhibitor of CNS axon outgrowth. J. Neurosci 31, 6481–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JS, et al. , 2013. Role of endogenous psychosine accumulation in oligodendrocyte differentiation and survival: implication for Krabbe disease. Brain Res. 1508, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, et al. , 2018. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature. 554, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wos M, et al. , 2016. Mitochondrial dysfunction in fibroblasts derived from patients with Niemann-pick type C disease. Arch. Biochem. Biophys 593, 50–59. [DOI] [PubMed] [Google Scholar]
- Xie C, et al. , 2017. AAV9-NPC1 significantly ameliorates Purkinje cell death and behavioral abnormalities in mouse NPC disease. J. Lipid Res 58, 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ren D, 2015. Lysosomal physiology. Annu. Rev. Physiol 77, 57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, et al. , 2012. Delta-Tocopherol reduces lipid accumulation in Niemann-pick type C1 and Wolman cholesterol storage disorders. J. Biol. Chem 287, 39349–39360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, et al. , 2014. A phenotypic compound screening assay for lysosomal storage diseases. J. Biomol. Screen 19, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, et al. , 2005. Hematopoietic cell transplantation ameliorates clinical phenotype and progression of the CNS pathology in the mouse model of late onset Krabbe disease. J. Neuropathol. Exp. Neurol 64, 565–575. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, et al. , 1994. The attenuated elevation of cytoplasmic calcium concentration following the uptake of low density lipoprotein in type C Niemann-pick fibroblasts. Biochem. Biophys. Res. Commun 198, 438–444. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, et al. , 2011. Positive feedback loop between PI3K-Akt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen. Cancer Res. 71, 4989–4997. [DOI] [PubMed] [Google Scholar]
- Yang F, et al. , 2019. Proteomics of the corpus callosum to identify novel factors involved in hypomyelinated Niemann-pick type C disease mice. Mol. Brain 12, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. , 2005. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. U. S. A 102, 13670–13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Lieberman AP, 2013. Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin. PLoS Genet. 9, e1003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaka M, Wenger DA, 2004. Psychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activation. Neurosci. Lett 358, 205–209. [DOI] [PubMed] [Google Scholar]
- Zancan I, et al. , 2015. Glucocerebrosidase deficiency in zebrafish affects primary bone ossification through increased oxidative stress and reduced Wnt/beta-catenin signaling. Hum. Mol. Genet 24, 1280–1294. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu Q, 2015. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 6, 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl R, et al. , 2010. Elevated sulfatide levels in neurons cause lethal audiogenic seizures in mice. J. Neurochem 112, 282–295. [DOI] [PubMed] [Google Scholar]