Figure 2.
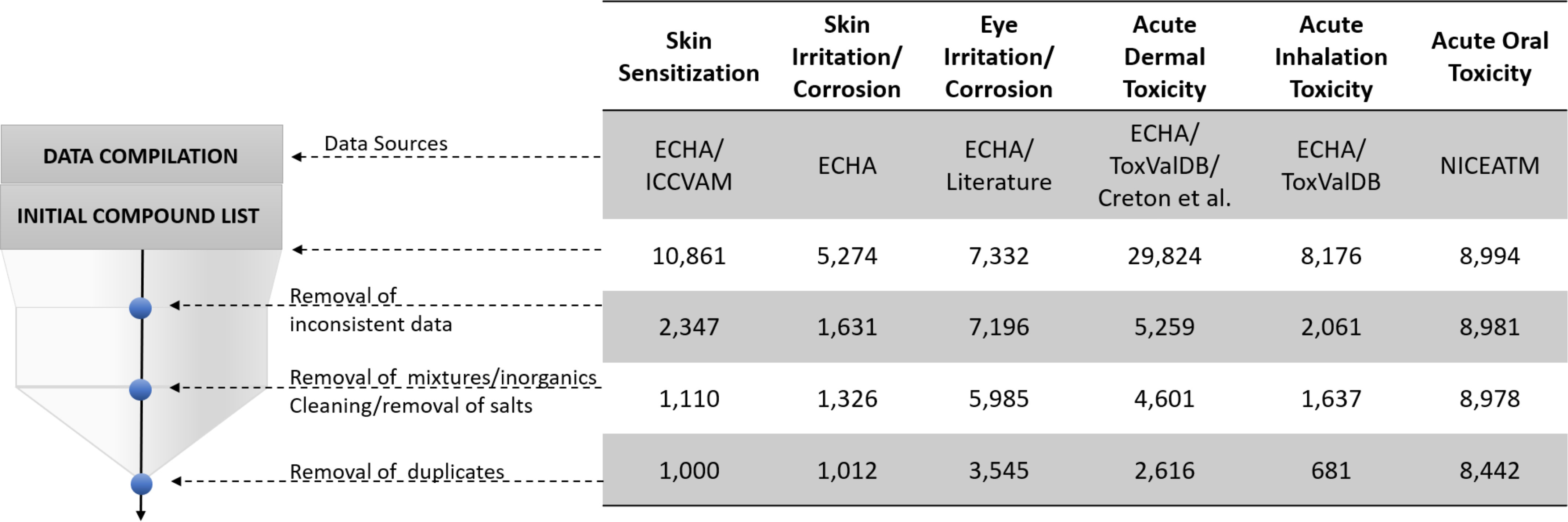
Summary of data curation steps. Data sources: ECHA (ECHA 2019; ECHA and OECD 2019), Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM 2013), ToxValDB (Judson 2018), and National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM; ICCVAM 2019).
