Figure 1.
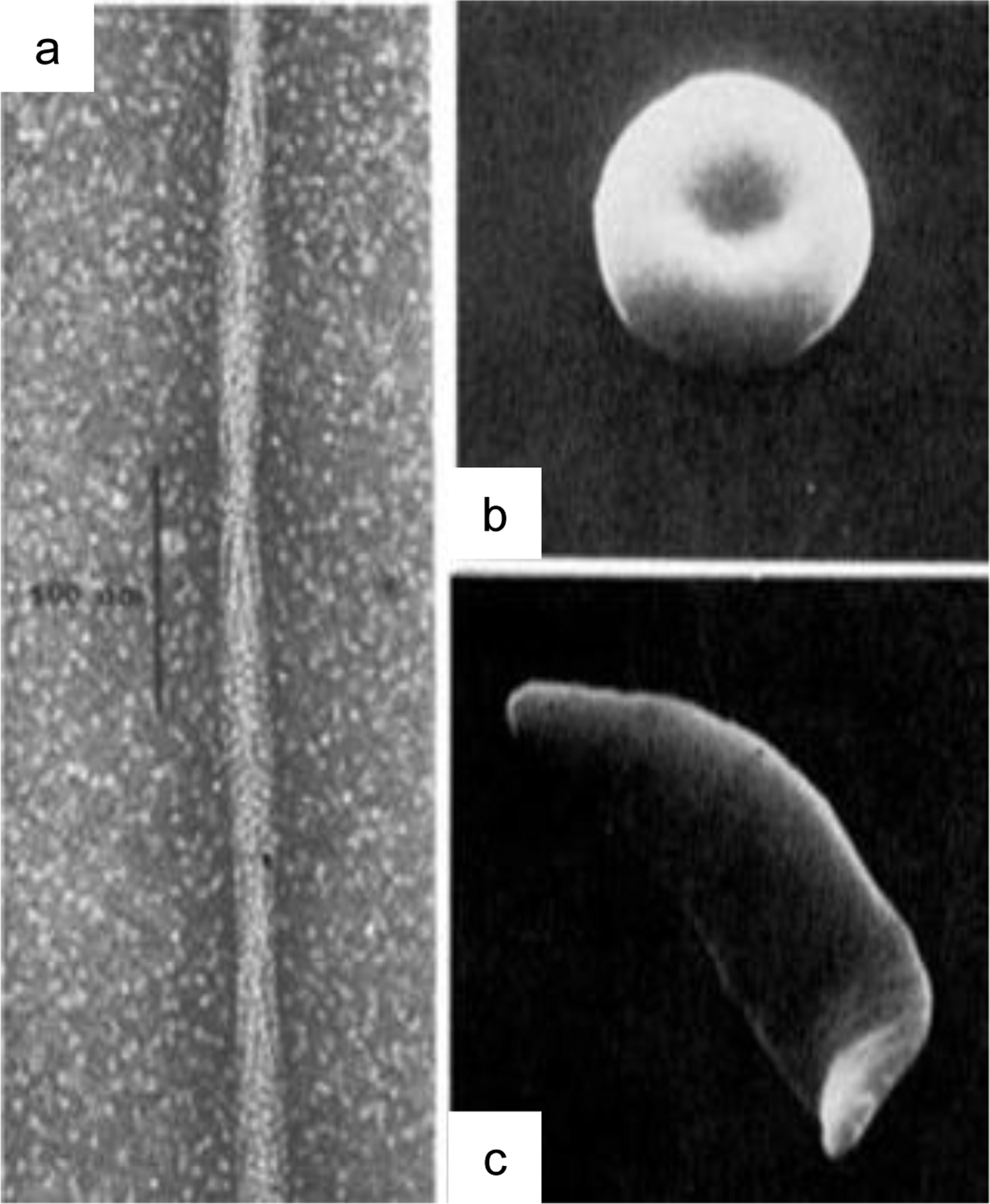
Polymerization of haemoglobin (Hb) drives sickle cell formation. (a) a single point mutation causes Hb to convert to a polymer in an oxygen and pH dependent manner. (b) normal shaped red blood cell. (c) Distortion of a red blood cell into a sickle shape. Reproduced with permission from Dykes, G., Crepeau, R. H. & Edelstein, S. J. Three-dimensional reconstruction of the fibres of sickle cell haemoglobin. Nature (1978) doi:10.1038/272506a04, and Eaton, W. A. & Hofrichter, J. Sickle Cell Hemoglobin Polymerization. Adv. Protein Chem. (1990) doi:10.1016/S0065-3233(08)60287-9.150
