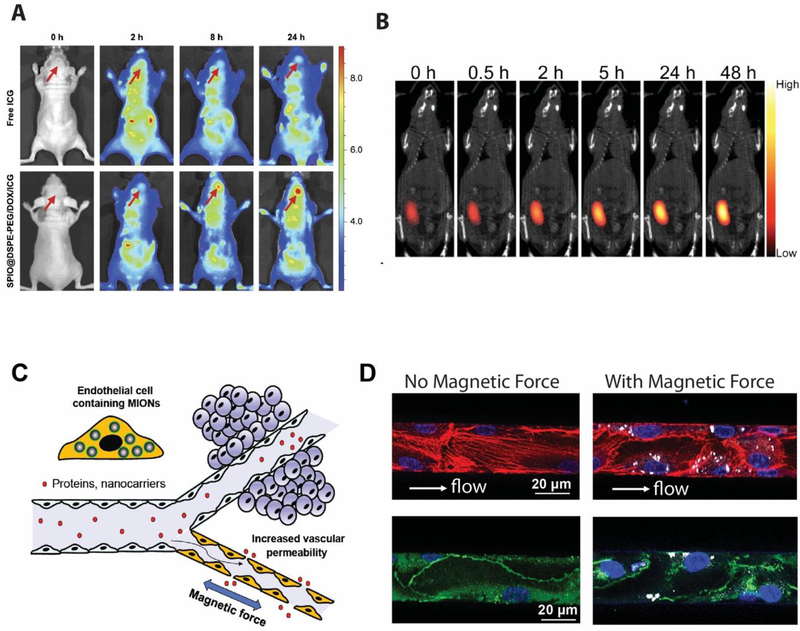
Figure 4. Application of MION for in vivo imaging and drug delivery.
A. Fluorescence images of glioma-bearing nude mice at different times after tail vein injection of free ICG and SPIO@DSPE-PEG/DOX/ICG NPs with arrows pointing at the location of glioma. B. Merged images of magnetic particle imaging (MPI, colored image) and X-ray computed tomography (CT, black and white) of an MDA-MB-231 tumor-bearing nude mouse injected intratumorally with iron oxide clusters containing Doxorubicin. C. A schematic illustration of the blood vessel that presents a major transport barrier to in vivo delivery by only allowing selective extravasation of solutes and small molecules, but not proteins and nanoparticles. When endothelial cells lining the interior surface of blood vessels have MIONs internalized, an applied magnetic field generates force in these cells, disrupting endothelial adherens junctions and increasing the vessel permeability, allowing proteins and nanocarriers to extravasate. D. MIONs are first delivered into endothelial cells in the microfluidic channels. After subjected to an applied magnetic field for 1 h after which the endothelialized channels were fixed and stained for actin and VE-cadherin. (Left) Without magnetic force, the endothelial actin filaments (red) were aligned along the flow direction and the adherens junctions were continuous (green). (Right) With applied magnetic forces, the number of actin filaments along the flow direction was reduced and the distribution of VE-cadherin became discontinuous and diffuse, indicating the disruption of adherens junctions at intercellular interfaces.