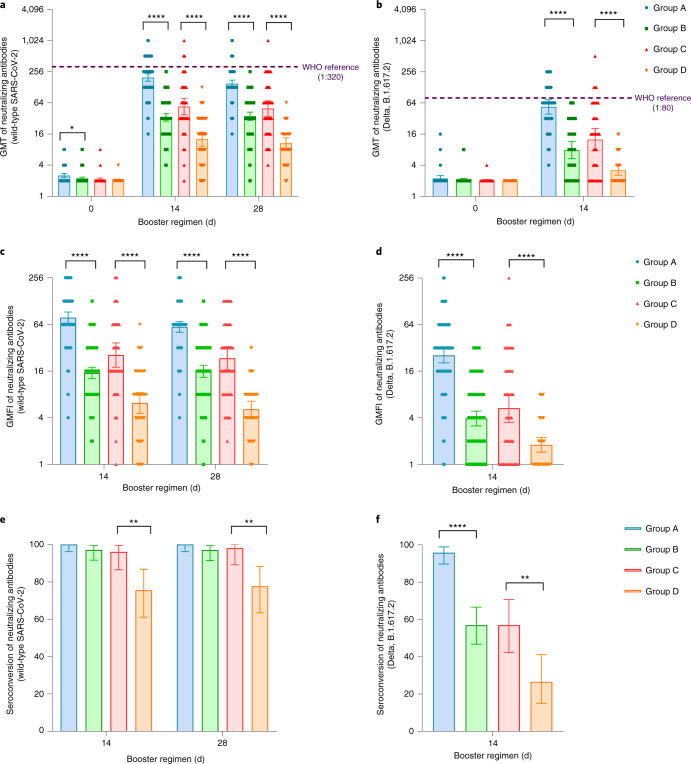
Fig. 2. Neutralizing antibodies to wild-type SARS-CoV-2 or the Delta variant before and after boosting.
GMTs of neutralizing antibodies to wild-type SARS-CoV-2 (a) or the Delta variant B1.617.2 (b). GMFI of neutralizing antibodies to wild-type SARS-CoV-2 (c) or the Delta variant B1.617.2 (d). Seroconversion of neutralizing antibodies to wild-type SARS-CoV-2 (e) or the Delta variant B1.617.2 (f). Error bars indicate 95% CIs. n, the number of participants included the intervention modified intention-to-treat cohort; seroconversion, proportion of participants with at least a fourfold increase in post-vaccination antibody levels compared to levels before the booster vaccination. Group A, primed with two doses of CoronaVac and given one dose of Convidecia (n = 96); group B, primed with two doses of CoronaVac and given one dose of CoronaVac (n = 102); group C, primed with one dose of CoronaVac and given one dose of Convidecia (n = 51); group D, primed with one dose of CoronaVac and given one dose of CoronaVac (n = 50). The analysis was based on the intervention modified intention-to-treat cohort. Measurements on day 0 were taken immediately before vaccination. The WHO reference (1,000 IU ml−1 in serum) is equivalent to a live viral neutralizing antibody titer of 1:320 against wild-type SARS-CoV-2 and 1:80 against the Delta variant B.1.617.2. P values result from comparison between the two treatment groups using t-tests for log-transformed antibody titers or two-sided χ2 tests for categorical data (group A versus group B and group C versus group D). No adjustments were made for multiple comparisons (group A versus group B and group C versus group D). For (e,f), the statistics are proportions of participants with seroconversion after the vaccination. **P < 0.005, ****P < 0.0001.