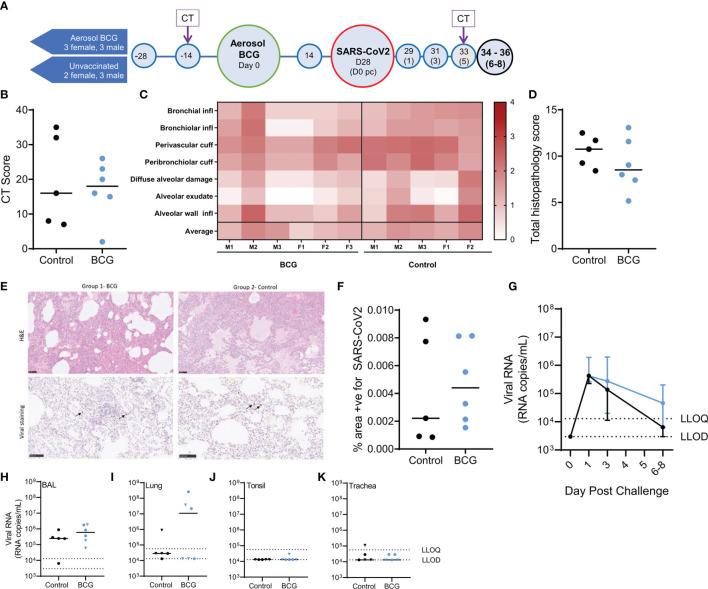
Figure 1.
Study timeline relative to aerosol BCG vaccination and disease outcome measures following SARS-CoV-2 challenge. (A) Rhesus macaques received aerosol BCG vaccination (n = six) at study day zero or were left as unvaccinated controls (n = six, reducing to five after day 28). Animals received SARS-CoV-2 by split intranasal and intrabronchial challenge (target dose 5 × 106 PFU) at day 28 and were monitored for up to 8 days post challenge (pc). Blue shaded circles represent procedures involving blood sample collection and application of immunological analyses; large circles represent key study events: vaccination and SARS-CoV-2 challenge, application of in vivo CT scanning is indicated. All animals were euthanized, and postmortem necropsies conducted upon completion of the study schedule (black circles) at days 34–36 (six–eight post challenge). (B) CT total score recorded at day five post-challenge. (C) Heatmap of pulmonary histopathology scores recorded in individual animals (males and females indicated). (D) Total lung histopathology scores. (E) Representative lung sections from animals from each group stained with either H&E or for expression of SARS-CoV-2 RNA. (F) Percentage area positive for SARS-CoV-2 staining in lung tissue sections. (G) SARS-CoV-2 viral RNA recovered from nasal swabs. Lower limits of detection (LLOD) and quantification (LLOQ) are indicated. (H–K) SARS-CoV-2 viral RNA recovered from (H) BAL samples collected at necropsy; (I) lung; (J) tonsil; and (K) trachea tissue samples. Plots show group median values (+/- IQR, plot G) with dots representing individual animals. Blue = BCG vaccinated, black = no vaccine.