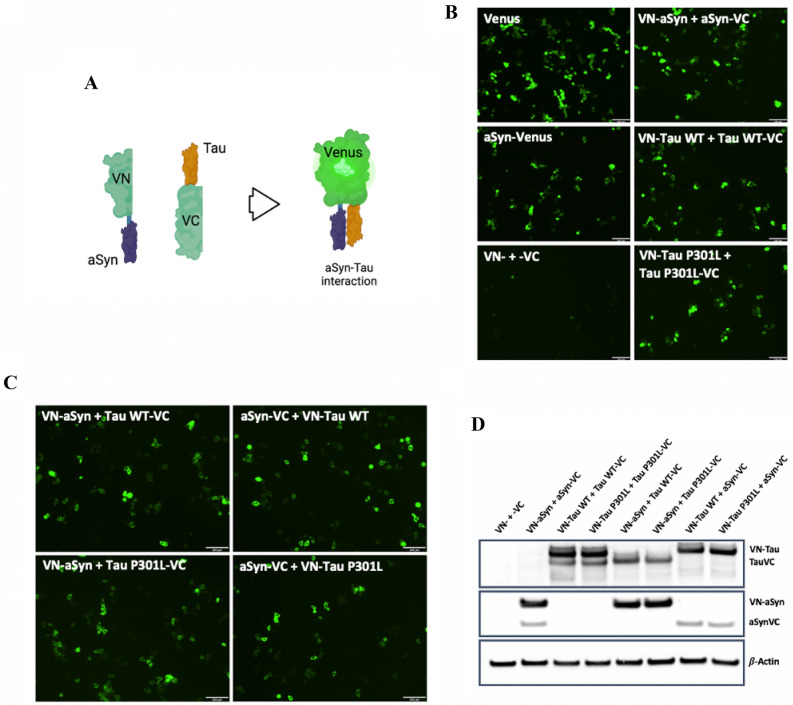
Figure 1.
Bimolecular Fluorescence Complementation (BiFC) assay confirms aSyn-Tau interaction in HEK293 cells. (A) Schematic representation of the BiFC assay. aSyn WT and/or Tau (WT or P301L) were expressed fused to one of the halves of the protein Venus (VN- or -VC). Complementation of the Venus halves due to interaction of aSyn with Tau promotes changes in the molecular conformation of the Venus protein leading to its emission of fluorescence (scheme created with BioRender.com). (B) Fluorescence emitted by Venus complementation 24 h post-transfection in HEK293 cells shows self-interaction of aSyn, Tau WT and Tau P301L. HEK293 cells were transiently (co-)transfected with different combinations of the BiFC constructs; the fluorescence emitted by Venus expression or complementation was recorded 24 h after transfection. Scale bar: 200 µm. (C) Fluorescence emitted by Venus complementation 24 h post-transfection in HEK293 cells shows interaction of aSyn with Tau (WT and P301L). HEK293 cells were transiently co-transfected with different combinations of the aSyn and Tau constructs, and the fluorescence emitted by Venus complementation was recorded 24 h after transfection. Scale bar: 200 µm. (D) Total levels of Tau and aSyn measured by western blot (WB). The levels of expression of aSyn and Tau in the cell lysate of HEK293 cells co-transfected with different BiFC constructs were measured by WB against total Tau and aSyn. β-Actin was used as loading control.