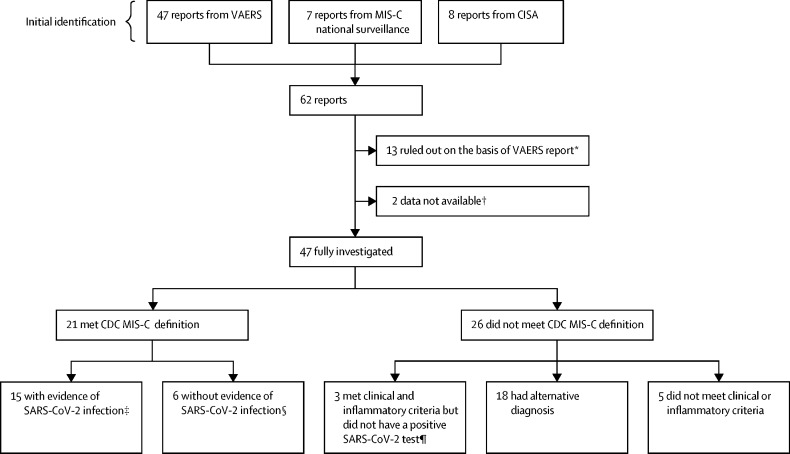
Figure.
Investigation of potential MIS-C in individuals who had received a COVID-19 vaccine
CDC=US Centers for Disease Control and Prevention. CISA=Clinical Immunization Safety Assessment. MIS-C=multisystem inflammatory syndrome in children. NAAT=nucleic acid amplification test. VAERS=Vaccine Adverse Event Reporting System. *If the individuals were the incorrect age or if MIS-C could be clearly ruled out on the basis of the VAERS report. †Two individuals were reported to MIS-C national surveillance but not to VAERS, and medical records were not obtained; both had reported MIS-C after one dose of COVID-19 vaccine, and both were positive for SARS-CoV-2 NAAT and IgG, but no further details were available; both clinically improved and were discharged home. ‡Defined as an illness meeting the CDC MIS-C clinical and inflammatory criteria with a positive NAAT or viral antigen test during or before MIS-C illness evaluation, or a positive anti-nucleocapsid antibody test during MIS-C illness evaluation. §Defined as an illness meeting the CDC MIS-C clinical and inflammatory criteria with negative NAAT and anti-nucleocapsid antibody tests and a positive anti-spike antibody test during MIS-C illness evaluation, with no known history of a positive SARS-CoV-2 test before MIS-C illness onset. ¶Three individuals with an illness after vaccination meeting the CDC MIS-C clinical and inflammatory criteria, a negative anti-nucleocapsid antibody test and negative NAAT test during MIS-C evaluation, and anti-spike antibody test not obtained.