Abstract
Introduction
Smokeless tobacco (SLT) causes significant harm to the oral cavity and is considered a risk factor for oral cancer. Various forms, products, and patterns of SLT are used across different populations. Many products, such as nicotine and betel nut, have addictive and carcinogenic properties. SLT use is associated with benign, premalignant, or malignant lesions. This study aimed to identify the characteristics of these oral lesions and their association with SLT exposure.
Materials and methods
This cross-sectional study, performed at our institution’s Faculty of Dentistry, included all the patients with a history of using SLT within a 5-year period at the oral medicine clinic. The patients’ demographic details were collected, and information regarding habit, duration, frequency, site of placement, and history of habit discontinuity were recorded. If a biopsy was performed, the diagnoses were also reported.
Results
Of the 59 patients included, 89.8% were male and 10.2% were female. SLT lesions in the oral cavity were usually focal lesions (76.3%). The most preferred placement site by SLT users was the mandibular posterior vestibule. Follow-up of SLT patients after quitting or clinical changes in the placement site showed a 92.8% regression or complete healing of the lesions. Of the 59 patients who underwent SLT, 18.6% were diagnosed with oral squamous cell carcinoma.
Conclusion
This study demonstrated a high percentage of remarkable regression or complete healing of SLT lesions related to early diagnosis and habit change. In contrast, 18.6% of the lesions progressed to SCC.
Keywords: Smokeless tobacco, Squamous cell carcinoma, Retrospective, Oral cancer
1. Introduction
Smokeless tobacco (SLT) includes tobacco that is used without burning (Al-Maweri et al., 2018a, Al-Maweri et al., 2018b, Alrashidi et al., 2018), and could be mixed with other ingredients or used alone (Al-Attas et al., 2014). SLT is consumed by chewing, dipping, and placing tobacco in the mouth vestibule. Globally, more than 300 million people consume SLT in 116 Asian, European, African, and American countries (Quadri et al., 2019, Warnakulasuriya and Straif, 2018). Various SLT forms, products, and patterns are used across different populations. In Saudi Arabia, 12.7% of the population were tobacco users in 2018, with men being more frequent users. (WHO, 2019).
SLT is composed of many products that have addictive properties and carcinogenic effects, such as nicotine, tobacco-specific nitrosamine, and different metals. The carcinogenic ingredients, such as betel nut, are typically enclosed in tobacco (Idris et al., 1998, Quadri et al., 2019). The mechanism of absorption of SLT products, mainly nicotine, in the oral cavity, varies according to the pH of the saliva in the mouth, which helps dissolve the material in the saliva (Stanisce et al., 2018).
SLT is associated with increased in teeth staining, dental caries, gingival diseases, and oral mucosal lesions, which could be benign, potentially malignant, or malignant (Al Agili and Park, 2013; Neville et al., 2015; Monika et al., 2020). SLT is also a risk factor for many oral lesions including squamous cell carcinoma (SCC) (Al-Attas et al., 2014, Bhar et al., 2017, Siddiqi et al., 2015). The potentially malignant oral lesions associated with SLT are leukoplakia, erythroplakia, and submucous fibrosis, while the main benign oral lesions is SLT hyperkeratosis (Khan et al., 2016). SLT keratosis is usually treated by habit discontinuity and education of the harmful effects of substance usage (Halboub et al., 2020). Fortunately, the keratotic changes are usually reversible within weeks of stopping the habit; however, if there is no change or with worsening lesion, a biopsy becomes necessary (Neville et al., 2015). Historically, patients were advised to change their site of placement, but this is no longer recommended because both sites would be exposed to the direct harmful effect of SLT (Halboub et al., 2020). In this study, we aimed to identify the characteristics and progression of both cancerous and non-cancerous oral lesions and their association with SLT exposure.
2. Materials and methods
A retrospective cross-sectional study was performed at King Abdulaziz University Faculty of Dentistry (KAUFD) for patients who had used SLT between January 2014 and December 2019 at an oral medicine clinic. Inclusion criteria were patients aged ≥18 years with confirmed use of SLT of any type. Patients who were already diagnosed with SCC at the first visit were excluded. The patients’ demographic details were collected from the medical records, and information regarding SLT type, duration, frequency, site of placement, and history of habit discontinuity were recorded. If a biopsy was performed, diagnoses were reported, and progression to SCC was also recorded. The study was approved by the KAUFD ethics committees (protocol number: 107-10-18), assuring compliance with the ethical principles for medical research involving human subjects.
2.1. Statistical analysis
Data are described as mean and standard deviation (SD) or frequency and percentages for continuous and categorical variables, respectively. The comparison between the two groups (SCC/non-SCC) according to the demographic, clinical, and diagnostic data was performed using the Pearson chi-square test, while the comparison of age, duration, and follow-up was assessed using Student’s t-test. All analyses were conducted using the SPSS version 24 for Windows (IBM Corp., Armonk, N.Y., USA), Stata Statistics Software (StataCrop, version 12), and Microsoft Office Excel (Windows, version 2016). Statistical significance was set at p < 0.05.
3. Results
3.1. Characteristics of the study population
Of 59 patients reported over a 5-year period as SLT users at the oral medicine clinics (Table 1). 89.8% (n = 53) were male (M:F ratio was 8.8:1). The age ranged from 18 to 79 years, with a mean of 41.9 (SD ± 13.3 years). Most SLT patients were non-Saudi (55.9%; n = 33). SLT lesions in the oral cavity were frequently detected as focal lesions (76.3%). The most preferred placement site by SLT users was the mandibular posterior vestibule (27.1%), followed by the maxillary anterior vestibule (23.7%). SLT was categorized as tobacco only, tobacco with alkalizing agents, tobacco with alkalizing agents and areca or betel, and more than one agent according to the National Cancer Institute and Centers for Diseases Control and Prevention (Hatsukami et al., 2014). The most used SLT category by patients was tobacco with alkaline agents including shamma and toombak (39%; n = 23). The detailed clinical information is presented in Table 1.
Table 1.
Characteristics of the study population including demographic, clinical, treatment, and follow up data comparing SCC and non-SCC patients.
| Parameters | All Population (n = 59) | SCC (n = 11) | Non-SCC (n = 48) | p-value | |
|---|---|---|---|---|---|
| Age (years) |
Mean ± SD Range |
41.90 ± 13.25 18–79 |
53.64 ± 12.2 36–75 |
39.15 ± 12.03 18–79 |
0.001* |
| Sex | Male | 53 (89.8%) | 7 (63.6%) | 46 (95.8%) | 0.001* |
| Female | 6 (10.2%) | 4 (36.4%) | 2 (4.2%) | ||
| Nationality*,† | Saudi | 20 (33.9%) | 2 (18.2%) | 18 (37.5%) | 0.591 |
| Non-Saudi | 33 (55.9%) | 5 (45.5%) | 28 (58.3%) | ||
| Medical history*,† | Healthy | 28 (47.5%) | 0 (0) | 28 (58.3%) | <0.001* |
| Chronic illness | 20 (33.9%) | 3 (27.3%) | 17 (35.4%) | ||
| Malignancy | 4 (6.8%) | 3 (27.3%) | 1 (2.1%) | ||
| Type of smokeless tobacco*,† | Non-tobacco | 8 (13.6%) | 2 (12.8%) | 6 (12.5%) | 0.233 |
| Tobacco alone | 1 (1.7%) | 0 (0) | 1 (2.1%) | ||
| Tobacco with alkalizing agents | 23 (39%) | 1 (9.1%) | 22 (45.8%) | ||
| Tobacco with alkalizing agents and areca or betel | 9 (15.3%) | 1 (9.1%) | 8 (16.7%) | ||
| More than one agent | 2 (3.4%) | 1 (9.1%) | 1 (2.1%) | ||
| Frequency of smokeless tobacco (per day)*,† | 1–3 times | 15 (25.4%) | 2 (18.2%) | 13 (27.1%) | 0.015* |
| 4–6 times | 14 (22%) | 2 (18.2%) | 13 (27.1%) | ||
| 6–10 times | 8 (5.1%) | 5 (45.5%) | 3 (6.3%) | ||
| More than 10 times | 5 (18.6%) | 1 (9.1%) | 10 (20.8%) | ||
| Duration (years) | Mean ± SD Range |
12.02 ± 8.91 1–40 |
9.0 ± 6.3 1–15 |
12.33 ± 9.14 1–40 |
0.257 |
| Location of the lesion *,† | No lesion | 3 (5.1%) | 0 (0) | 3 (6.3%) | 0.003* |
| Upper labial vestibule unilateral | 14 (23.7%) | 0 (0) | 14 (29.2%) | ||
| Lower labial vestibule unilateral | 8 (13.6%) | 0 (0) | 8 (16.7%) | ||
| Labial vestibule bilateral | 2 (3.4%) | 0 (0) | 2(4.2%) | ||
| Buccal bilateral | 5 (8.5%) | 0 (0%) | 5 (10.4%) | ||
| Buccal unilateral | 16 (27.1%) | 8 (72.7%) | 8 (16.7%) | ||
| Palate | 1 (1.7%) | 0 (0) | 1 (2.1%) | ||
| Floor of the mouth | 3 (5.1%) | 2 (18.2%) | 1 (2.1%) | ||
| Adjusted size (cm) | Mean ± SD Range |
2.56 ± 1.37 mm 0.4–6 |
4.0 ± 1.63 2–6 |
2.3 ± 1.2 0.4–5 |
0.001* |
| Demarcation*,† | Poorly demarcated | 25 (42.4%) | 11 (100%) | 14 (29.2%) | <0.001* |
| Well demarcated | 22 (37.3%) | 0 (0) | 22 (45.8%) | ||
| Biopsy | No | 41 (69.5%) | 0 (0) | 41 (85.4%) | <0.001* |
| Yes | 18 (30.5%) | 11 (100%) | 7 (14.6%) | ||
| Change/stopping of the habit*,† | No | 15 (25.4%) | 0 (0) | 15 (31.3%) | 0.117 |
| Yes | 20 (33.9%) | 3 (27.3%) | 17 (35.4%) | ||
| Follow-up lesion change (only the cases with follow up confirmation were counted) † | No | 6 (10.2%) | 0 (0) | 6 (12.5%) | 0.009* |
| Healed | 13 (22%) | 2 (18.2%) ** | 11 (22.9%) | ||
| Remarkable regression | 5 (8.5%) | 0 (0) | 5 (10.4%) | ||
| Died | 2 (3.4%) | 2 (18.2%) | 0 (0) | ||
| Recurrent | 1 (1.7%) | 0 (0) | 1 (2.1%) | ||
| Histopathology | No | 42 (71.2%) | 0 (0) | 42 (87.5%) | <0.001* |
| Hyperkeratosis | 6 (10.2%) | 0 (0) | 6 (12.5%) | ||
| SSC | 11 (18.6%) | 11 (100%) | 0 (0) | ||
| Color* | White | 30 (50.8%) | 2 (18.2%) | 28 (58.3%) | <0.001* |
| Red | 8 (13.6%) | 8 (72.7%) | 0 (0) | ||
| Red and white | 3 (22%) | 1 (9.1%) | 2 (4.7%) | ||
| Black | 1 (1.7%) | 0 (0) | 1 (2.1%) | ||
| Grayish white | 8 (13.6%) | 0 (0) | 8(16.7%) |
SCC, squamous cell carcinoma; SD, standard deviation.
Statistically significant p-value.
follow up record after surgical treatment.
Some data not available.
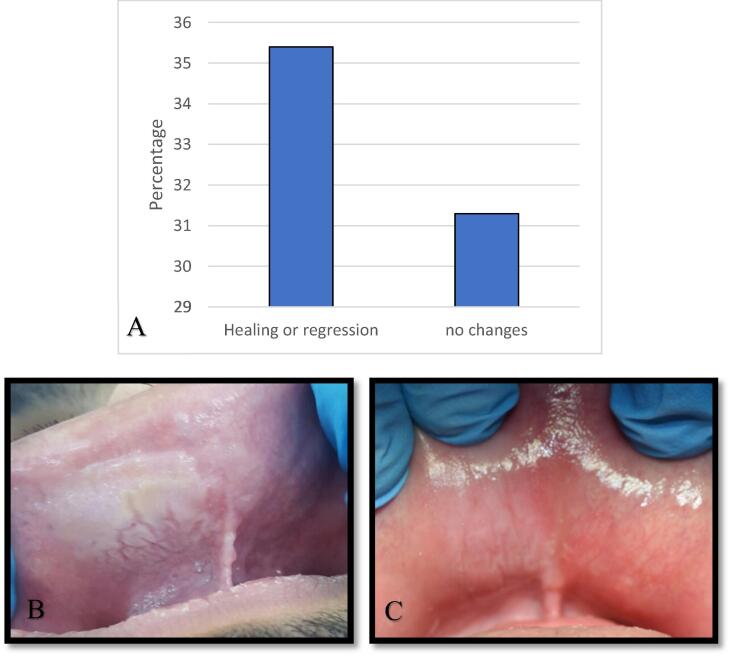
Most patients had non-cancerous SLT lesions was 81.4% (n = 48). These patients were advised to quit their habit or change the SLT placement site. Only 35.4% (n = 17) admitted changing the frequency of use, quitting, or changing the placement site, while 31.3% (n = 15) did not (no follow-up records, n = 16) (Fig. 1). Follow-up of these lesions after quitting or changing the placement site showed a 92.8% regression or complete healing of the SLT lesions. Among the 59 patients who used SLT, 18.6% (n = 11) were diagnosed with SCC. These had no documented follow-up in our system, because they were mostly referred for treatment in other centers.
Fig. 1.
Follow up of the non-cancerous SLT lesions, A- Bar chart showing the patients' follow up for non-cancerous SLT lesions. B- Patients with smokeless tobacco keratosis located at the right side of the labial mucosa. C- Follow up after 10 weeks, the patient quit the habit, and the lesion is almost resolved. (SLT, smokeless tobacco).
3.2. Comparison between SCC and non-SCC groups
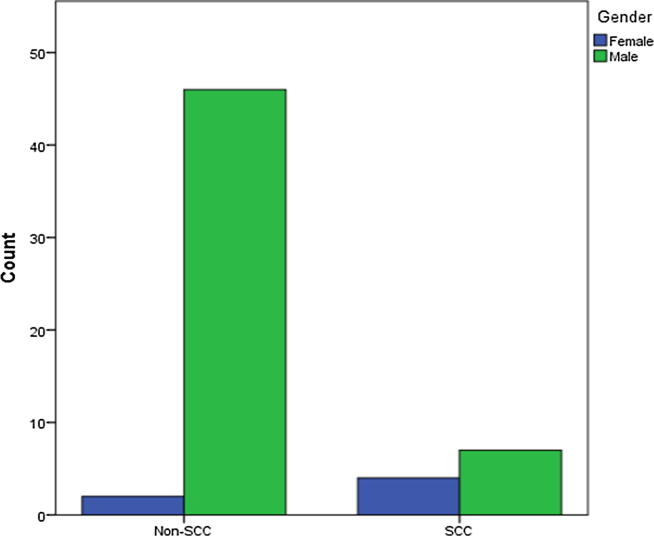
The patients with SCC were significantly older (mean age; 53.64, SD ± 12.2 years) than the non-SCC patients (mean age, 39.15, SD ± 12.03 years) (p = 0.001). Males had a considerably higher risk of SCC than females (p = 0.001) (Fig. 2). Regarding nationality, there was no significant difference between Saudi and non-Saudis in developing SCC. Overall, 54.6% (p < 0.001) of SCC patients presented with chronic medical illnesses or malignancies. No significant difference was found between SLT types in developing SCC; all agents yielded the same cancerous changes (p = 0.233). Moreover, no significant difference occurred between the SCC and non-SCC groups according to duration of use (p = 0.483), although there was significant difference in the frequency of use (p = 0.015). The frequency of SCC was higher in patients with lesions in the mandibular posterior vestibule (72.7%) than those with lesions on the floor of the mouth (18.2%). Furthermore, the clinical presentations of SCC displayed white/red and poorly demarcated lesions.
Fig. 2.
Gender distribution among SCC and Non-SCC groups. A bar chart showing gender distribution in SCC and non-SCC SLT users. (SCC, squamous cell carcinoma; SLT, smokeless tobacco).
4. Discussion
The prevalence of SLT use varies among countries and ranged from 1.2% to 7.5% in youths and 0.4% to 17.9% in adults (WHO, 2019). Southeast Asian countries showed the highest prevalence (17.9%) of SLT users as they replaced regular tobacco smoking with SLT, in a misconception, one with a less harmful effect than other alternative habits (Khan et al., 2016, Suliankatchi et al., 2019, WHO, 2019). The prevalence of SLT according to a Saudi health interview survey in 2013 in adults (15 years or older) was 0.9%, although the WHO predicted an increase in the prevalence of tobacco use and smoke to 19.2% by 2025 (Ministry of Health (MOH) and Arabia, 2013, WHO, 2019).
SLT use was linked to an increased risk of several health problems, including oral cancer (Stanisce et al., 2018, Warnakulasuriya and Ralhan, 2007). In the United States, the relative risk of oral and oropharyngeal cancer when comparing SLT users to non-users ranged from 1.65 to 2.6. A large study including 32 countries found an estimated pooled non-specific relative risk for oral cancers in SLT users of 3.43 (Siddiqi et al., 2015). Our study revealed that 18.6% of SLT users had oral SCC; however, this figure is comparatively lower than the 45.3% reported previously by Idris et al. (Idris et al., 2016) in Jazan, surprisingly with female predominance (M:F ratio of 1:1.9). This contrasts with the current study, which has a distinct male predominance (M:F ratio of 1.75:1). This might be because around half of the population in Jazan uses shamma, and females showed double the rate of use of males (Idris et al., 2016, Itumalla and Aldhmadi, 2020). Moreover, the study size of SLT users (n = 59) might affect the validity of the sex distribution, since the number of female SLT patients (n = 6) was much lower in comparison to male SLT patients (n = 53). This large difference might not reflect the real ratio in Jeddah. Previously a study conducted in Riyadh, reported that 16.4% of oral cancer cases were associated with shamma (Amer et al., 1985).
The risk of developing SCC increases with age, as this study concluded that the mean age of SCC patients was 53.64 years, which is similar to a study conducted by Allard et al. that reported a mean age of SLT patients with malignant lesions of 58 years (Allard et al., 1999). SLT (especially shamma) is reported to be used among adolescents in Saudi Arabia; however, in this study, the youngest patient was 18 years old. A study conducted in Jeddah, Saudi Arabia, which targeted middle school males in a low socioeconomic area, showed that the mean age of users was 15.7 years. These young males reported starting the habit as young as 12.9 years old (Al Agili and Park, 2013). These young users chewed tobacco about five times daily, leaving it for ten minutes each time, which is slightly similar to that in our study where 47.4% of the patients chewed SLT one to six times daily with a mean duration of 12.2 years. The mean duration of SLT use reported by Amer et al. was 27.1 years (Amer et al., 1985). In our study, the duration of SLT use was not associated with increased risk of cancer; however, an association was found with the frequency of SLT use. This result might be affected by the missing data of some patients. Other studies have proved dose dependently that time, contact, and frequency of use would increase the risk in these patients (Monika et al., 2020, Niaz et al., 2017, Quadri et al., 2019). The most common location for SLT lesions was the mandibular posterior vestibule in the current study, in accordance with previous studies where the patients chewed, dipped, and placed tobacco in the maxillary or mandibular vestibule or mucobuccal folds (Al Agili and Park, 2013, Idris et al., 2016).
The risk could also vary according to the type of SLT (Alrashidi et al., 2018). SLT types differ among SLT users; but those commonly consumed in Saudi are shamma, toombak, and qat. A meta-analysis showed that shamma users have a 39 times increased risk of developing oral cancer compared to non-users (Quadri et al., 2019). A study in King Faisal Specialist Hospital and Research Center in Riyadh found that 49% of oral cancers were related to the use of shamma, while another study in Jazan revealed that 45.3% of shammah users were diagnosed with SCC (Amer et al., 1985, Idris et al., 2016). Shammah and toombak are categorized as tobacco with alkylating agents, which was the most frequently used among our patients (39%) (Hatsukami et al., 2014). Tombaak products are commonly used in Sudan while snus, Catha edulis (Qat), and Paan/betel quid are the most commonly used in Sweden, Yemen, and South Asian Countries, respectively (Al-Maweri et al., 2018b, Idris et al., 1998, Niaz et al., 2017).
The effect of SLT starts by the dissolving of its constituents in the saliva and subsequent local absorption (Tomar and Henningfield, 1997). The components of SLT vary, with some considered to be carcinogenic (e.g., nitrosamine and nicotine) (Niaz et al., 2017, Warnakulasuriya and Straif, 2018). SLT components can affect human epithelial cells and fibroblasts by increasing the production of reactive oxygen species, cell turnover, collagen synthesis, and gingival blood flow or DNA damage, which causes oral mucosal changes and may progress to SCC (Warnakulasuriya and Straif, 2018). The clinical presentation varies from grayish-white ill-demarcated lesions, velvety in appearance to sometimes red with ulceration. This presentation is mainly based on cellular changes, where white to gray appearance is mostly due to the increase in the thickness of the keratin layer and/or acanthosis with keratinocyte edema. Additionally, some types may cause an increase in the fibrous tissue composition of the connective tissue and collagen sclerosis (Müller, 2019). Recent research has shown that various types of SLT harbor bacteria that can play a role in the carcinogenicity (Halboub et al., 2020, Monika et al., 2020). In the current study, the most common clinical presentation was well-demarcated white and grayish lesions. Furthermore, most of our patients were healthy; but diabetes and hypertension were reported along with other diseases with no statistical significance. Alattas et al. showed a strong association between the presence of SLT lesions and diabetes mellitus (Al-Attas et al., 2014).
Generally, SLT lesions should be treated by habit discontinuity which show resolution within 6 weeks to 6 months; otherwise, a biopsy is indicated (Müller, 2019). SLT lesions show an increased risk of progression to potential malignant lesions and oral cancer ((Neville et al., 2015); Müller, 2019). If the patient presents with a red component or ulceration, this should raise the alarm for biopsy of the lesion to rule out malignant transformation. In this study, 92.8% of patients showed regression or complete healing of the non-cancerous SLT lesions after quitting, although some of the patients changed their placement site instead of completely quitting. Changing the placement site of the SLT is not advised because of the increase in direct exposure at a different location; however, in case of refusing to quit this habit immediately, this action may help in reducing the occurrence of cancer. Further studies are needed to determine the duration of SLT useage associated with the development of oral cancerous lesions by robust design methodology. In our study, the regression and disappearance of the SLT lesion by quitting of the SLT habit was promising, however, despite this, 18.6% of the SLT users were diagnosed with oral SCC. Many previous studies in Saudi have shown a higher prevalence of oral cancer in association with SLT (Allard et al., 1999, Amer et al., 1985, Idris et al., 2016, Quadri et al., 2019). There is overwhelming evidence demonstrating the carcinogenicity of SLT in the oral cavity; thus, community education, strict cessation protocols, and recall visits should be applied to minimize the development of oral cancers (Stanisce et al., 2018). In the past, smoking cessation campaigns were mainly focused on cigarette smoking, leading smokers to replace regular smoking with SLT use (Itumalla and Aldhmadi, 2020, Suliankatchi et al., 2019). As the evidence grows against SLT, more public health awareness and education about the harmful effects of SLT are necessary.
We acknowledge some limitations to this study that may hinder its generalizability, including, the small sample size, heterogeneous nature of included patients and variaations of the lesion types, sex distribution, and mostly non-Saudis with a limited age range.
5. Conclusions
SLT lesions may start as benign lesions that can be reversed if the habit is stopped. The present study demonstrated a remarkable regression or healing of these lesions. Continuity of SLT use is harmful and associated with a risk of SCC occurrence in approximately 18%–45% of cases according to the current data and the literature discussed. There is a need to develop a plan to support efforts to prevent SLT usage in Saudi Arabia and prevent the potential harm.
Ethical Statement
Ethical approval was obtained from King Abdulaziz University, Faculty of Dentistry ethics committees (protocol number: 107-10-18).
CRediT authorship contribution statement
Nada Binmadi: Conceptualization, Validation, Writing – original draft, Writing – review & editing, Supervision, Project administration. Louae Harere: Methodology, Investigation, Data curation. Ajwad Mattar: Methodology, Investigation, Data curation. Suad Aljohani: Validation, Writing – review & editing. Nada Alhindi: Methodology, Formal analysis. Sarah Ali: Writing – review & editing. Soulafa Almazrooa: Conceptualization, Methodology, Validation, Resources, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
Authors would like to thank SevoClin CRO for statistical services.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Attas S.A., Ibrahim S.S., Amer H.A., Darwish Z.-E.-S., Hassan M.H. Prevalence of potentially malignant oral mucosal lesions among tobacco users in Jeddah, Saudi Arabia. Asian Pac. J. Cancer Prev. 2014;15:757–762. doi: 10.7314/apjcp.2014.15.2.757. [DOI] [PubMed] [Google Scholar]
- Al-Maweri S.A., Al-Jamaei A., Saini R., Laronde D.M., Sharhan A. White oral mucosal lesions among the Yemeni population and their relation to local oral habits. J. Investig. Clin Dent. 2018;9 doi: 10.1111/jicd.12305. [DOI] [PubMed] [Google Scholar]
- Al-Maweri S.A., Warnakulasuriya S., Samran A. Khat (Catha edulis) and its oral health effects: An updated review. J. Investig. Clin. Dent. 2018;9 doi: 10.1111/jicd.12288. [DOI] [PubMed] [Google Scholar]
- Al Agili D.E., Park H.-K. Oral health status of male adolescent smokeless tobacco users in Saudi Arabia. East. Mediterr. Heal J = La Rev sante la Mediterr Orient = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2013;19:711–719. [PubMed] [Google Scholar]
- Allard W.F., DeVol E.B., Te O.B. Smokeless tobacco (shamma) and oral cancer in Saudi Arabia. Community Dent. Oral Epidemiol. 1999;27:398–405. doi: 10.1111/j.1600-0528.1999.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Alrashidi A.G., Alrashidi T.G., Alrashedi S.A., et al. Epidemiologic pattern and types of oral smokeless tobacco usage in Saudi Arabia. J. Contemp. Dent. Pract. 2018;19:456–462. [PubMed] [Google Scholar]
- Amer M., Bull C.A., Daouk M.N., McArthur P.D., Lundmark G.J., El Senoussi M. Shamma usage and oral cancer in Saudi Arabia. Ann. Saudi Med. 1985;5:135–140. doi: 10.5144/0256-4947.1985.135. [DOI] [Google Scholar]
- Bhar D., Bhattacherjee S., Mukherjee A., Sarkar T.K., Dasgupta S. Utilization of safe drinking water and sanitary facilities in slum households of Siliguri, West Bengal. Indian J. Public Health. 2017;61:248–253. doi: 10.4103/ijph.IJPH_345_16. [DOI] [PubMed] [Google Scholar]
- Halboub, E., Al-Ak’hali, M.S., Alamir, A.H., et al., 2020. Tongue microbiome of smokeless tobacco users. BMC Microbiol. 20, 201. 10.1186/s12866-020-01883-8 [DOI] [PMC free article] [PubMed]
- Hatsukami, D., Zeller, M., Gupta, P., Parascandola, M. and Asma, S., 2014. Smokeless tobacco and public health: A global perspective. Cancer Institute. Natl. Dis. Control. Centers. Dis. Control. Prev. Dep. Heal .Us. Serv Hum. 14–7983.
- Idris A.M., Ibrahim S.O., Vasstrand E.N., et al. The Swedish snus and the Sudanese toombak: are they different? Oral Oncol. 1998;34:558–566. doi: 10.1016/s1368-8375(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Idris A.M., Vani N.V., Saleh S., et al. Relative frequency of oral malignancies and oral precancer in the biopsy service of Jazan province, 2009–2014 17, 2009–2014. Asian Pac. J. Cancer Prev. 2016;17:519–525. doi: 10.7314/apjcp.2016.17.2.519. [DOI] [PubMed] [Google Scholar]
- Itumalla R., Aldhmadi B. Combating tobacco use in Saudi Arabia: A review of recent initiatives. East. Mediterr. Heal. J. 2020 doi: 10.26719/emhj.20.019. [DOI] [PubMed] [Google Scholar]
- Khan Z., Khan S., Christianson L., Rehman S., Ekwunife O., Samkange-Zeeb F. Smokeless tobacco and oral potentially malignant disorders in South Asia: A protocol for a systematic review. Syst. Rev. 2016;5:1–7. doi: 10.1186/s13643-016-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health (MOH), Saudi Arabia, 2013. Saudi Health Interview Survey. Riyadh.
- Monika, S., Dineshkumar, T., Priyadharini, S., Niveditha, T., Sk, P., Rajkumar, K., 2020. Smokeless tobacco products (STPs) harbour bacterial populations with potential for oral carcinogenicity. Asian Pac. J. Cancer Prev. 21, 815–824. 10.31557/APJCP.2020.21.3.815 [DOI] [PMC free article] [PubMed]
- Müller S. Frictional keratosis, contact keratosis and smokeless tobacco keratosis: Features of reactive white lesions of the oral mucosa. Head NeckPathol. 2019;13:16–24. doi: 10.1007/s12105-018-0986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville, D., Douglas, D., Allen, C., Bouquot, J., 2015. Oral and Maxillofacial Pathology, 5th ed. W B Saunders Co.
- Niaz K., Maqbool F., Khan F., Bahadar H., Ismail Hassan F., Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol. Health. 2017;39 doi: 10.4178/epih.e2017009. e2017009–e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M.F.A., Tadakamadla S.K., John T. Smokeless tobacco and oral cancer in the Middle East and North Africa: A systematic review and meta-analysis. Tob. Induc. Dis. 2019;17:56. doi: 10.18332/tid/110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi K., Shah S., Abbas S.M., et al. Global burden of disease due to smokeless tobacco consumption in adults: Analysis of data from 113 countries. BMC Med. 2015;13:194. doi: 10.1186/s12916-015-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisce L., Levin K., Ahmad N., Koshkareva Y. Reviewing smokeless tobacco epidemiology, carcinogenesis, and cessation strategy for otolaryngologists. Laryngoscope. 2018;128:2067–2071. doi: 10.1002/lary.27104. [DOI] [PubMed] [Google Scholar]
- Suliankatchi R.A., Sinha D.N., Rath R., et al. Smokeless tobacco use is “replacing” the smoking epidemic in the South-East Asia Region. Nicotine Tob. Res. 2019;21:95–100. doi: 10.1093/ntr/ntx272. [DOI] [PubMed] [Google Scholar]
- Tomar S.L., Henningfield J.E. Review of the evidence that pH is a determinant of nicotine dosage from oral use of smokeless tobacco. Tob. Control. 1997;6:219–225. doi: 10.1136/tc.6.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnakulasuriya K.A.A.S., Ralhan R. Clinical, pathological, cellular and molecular lesions caused by oral smokeless tobacco–a review. J. Oral Pathol. Med. 2007;36:63–77. doi: 10.1111/j.1600-0714.2007.00496.x. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya S., Straif K. Carcinogenicity of smokeless tobacco: Evidence from studies in humans & experimental animals. Indian J. Med. Res. 2018;148:681–686. doi: 10.4103/ijmr.IJMR_149_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2019. WHO global Report on Trends in Prevalence of Tobacco Smoking 2000–2025, third ed., World Health Organisation. World Health Organization, Geneva.