Figure 1. Recruitment, Randomization, and Follow-up of Patientsa.
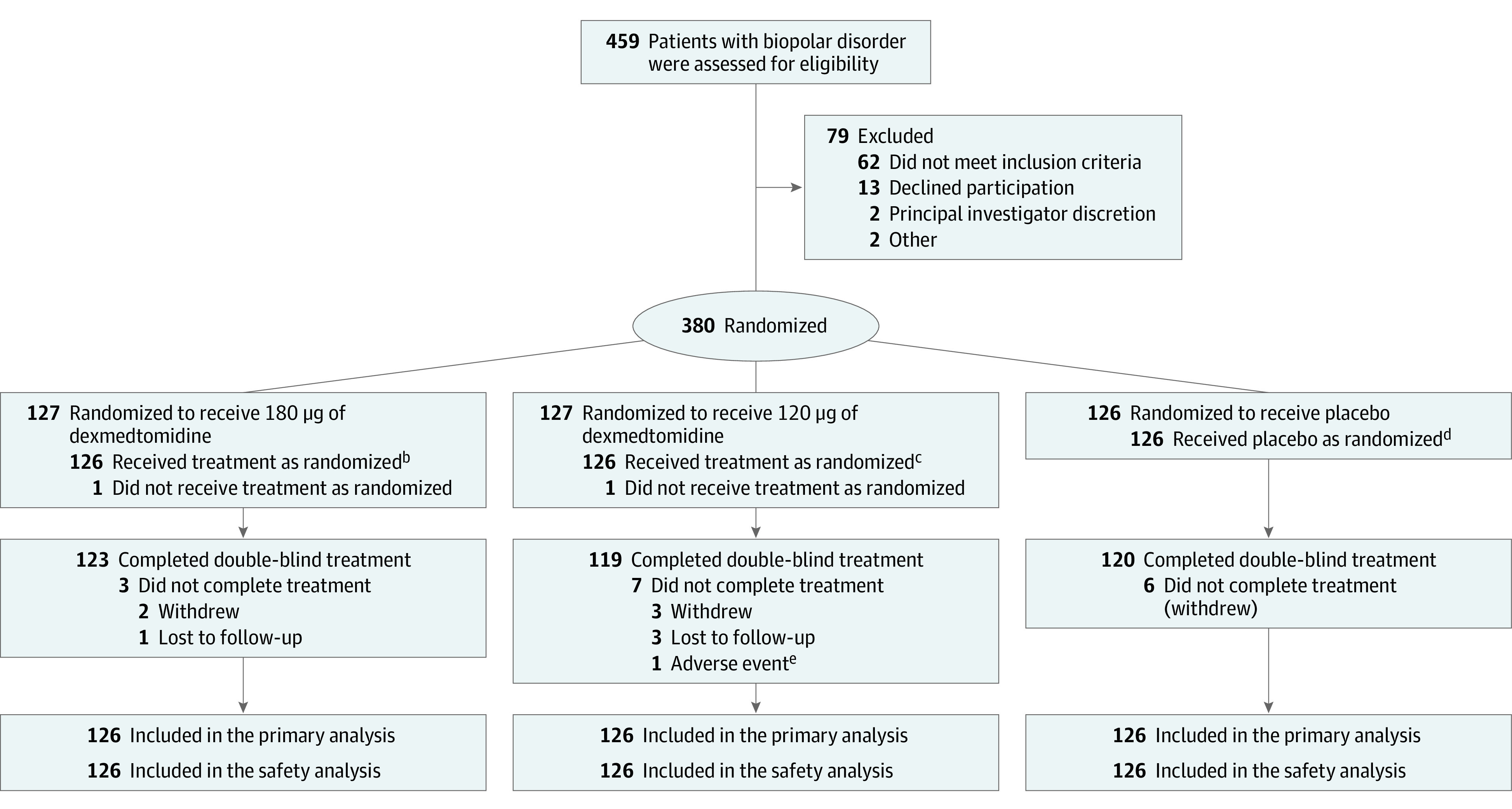
aThis was a single-dose study, and the safety, full analysis, and per-protocol data sets all contained 378 patients—126 in each group. There were no missing data for the primary or secondary outcome measures. One patient each from 180 μg and 120 μg groups did not receive a dose of medication, but all other discontinuations occurred after dosing and primary and secondary end point assessments.
bOne hundred thirteen patients (89.7%) received only 1 dose; 5 (4%), 2 doses; and 8 (6.3%), 3 doses.
cNinety-six patients (76.2%) received only 1 dose; 18 (14.7%), 2 doses; and 12 (9.5%), 3 doses.
dSixty-eight patients (54%) received only 1 dose; 29 (23.0%), 2 doses; and 29 (23%), 3 doses.
eDue to acute agitation on day 7 that was judged to be unrelated to study drug.
