Table 3:
Substrate scope of the asymmetric diarylation of vinylarenes.[a]
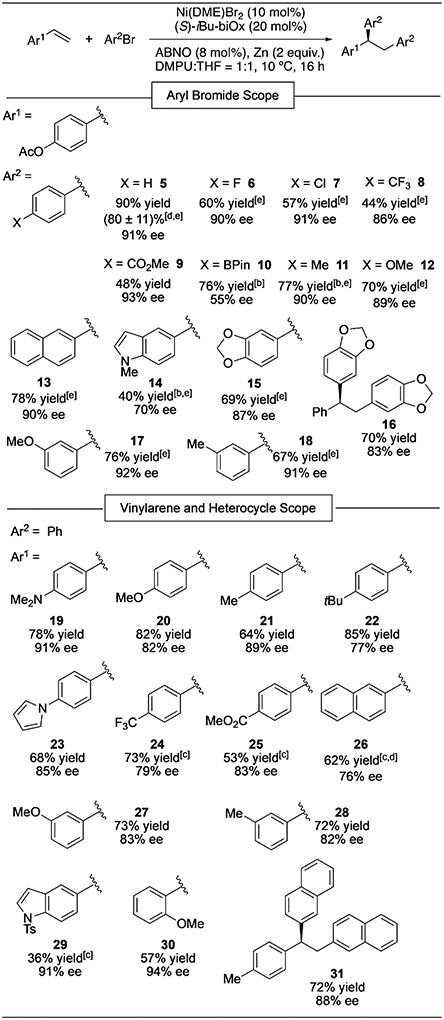
|
Reaction conditions: Vinylarene (0.2 mmol, 1 m), ArBr (4 m). Yields determined by 1H NMR analysis using mesitylene as an internal standard; ee values determined by HPLC analysis on a chiral stationary phase. The absolute stereochemistry was assigned for 29 based on X-ray crystallography, while those of all other products were assigned by analogy.
Vinylarene (0.5 m).
With 20 mol % Ni(DME)Br2, 40 mol % iBu-biOx, and 16 mol % ABNO.
Yield of isolated product. The error bar is based on four duplicate experiments.
The product was isolated as the corresponding phenol after deacylation with aqueous NaOH.
