Optimization of the crossed [2+2] photocycloaddition of 2-(allyloxy)cyclohex-2-enone (4a) to product 5a catalyzed by chiral Lewis acid 6.
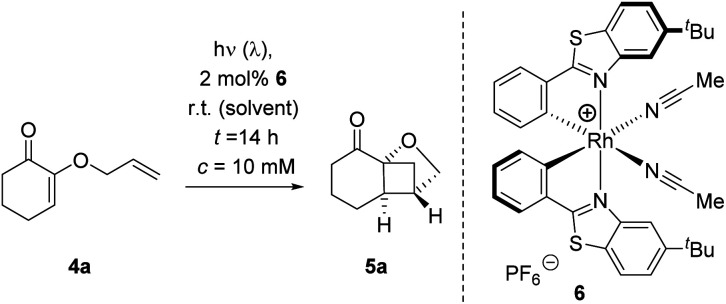
| |||||
|---|---|---|---|---|---|
| Entrya | Solvent | λ [nm] | Conv.b [%] | Yieldc [%] | % eed |
| 1 | MeCN | 425 | 2 | — | — |
| 2 | PhMe | 425 | 11 | 11 | 15 |
| 3 | THF | 425 | 11 | 11 | 20 |
| 4 | CH2Cl2 | 425 | 85 | 22 | 87 |
| 5 | DCE | 425 | 83 | 61 | 91 |
| 6e | DCE | 425 | 91 | 68 | 87 |
| 7f | DCE | 425 | 95 | 86 | 85 |
| 8 | DCE | 437 | 93 | 86 | 92 |
| 9e | DCE | 437 | 73 | 55 | 90 |
| 10g | DCE | 437 | 79 | 64 | 94 |
| 11h | DCE | 437 | 89 | 78 | 87 |
| 12i | DCE | 437 | 72 | 70 | 87 |
All reactions were performed under anaerobic conditions employing a light emitting diode (LED) in a previously described set-up.19
The conversion (conv.) was determined by NMR analysis.
All yields refer to isolated material.
Enantiomeric excess (ee) as determined by GLC analysis on a chiral stationary phase.
The reaction was performed in the presence of air.
The reaction time was 24 hours.
The catalyst loading was 4 mol%.
The reaction was performed at 0 °C.
The substrate concentration was 4 mM.
