Abstract
Objectives
The United States (US) FDA, European Surveillance of Veterinary Antimicrobial Consumption (ESVAC), Public Health Agency of Canada (PHAC) and World Organisation for Animal Health (OIE) established methodologies that characterize antimicrobial sales for use in food animals by adjusting the sales by animal biomass. Our aim was to review and compare these methodologies on US-specific data.
Methods
Annual antimicrobial sales for cattle, swine, chickens and turkeys in the USA between 2016 and 2018 were adjusted by the FDA, ESVAC, PHAC and OIE methodologies. To better understand the advantages and disadvantages of the four methodologies, their biomass denominators were compared regarding the level of detail accounted for in the estimated US livestock biomass, their ability to observe temporal trends in animal biomass within a country and practicality in biomass estimation for comparing antimicrobial sales across countries.
Results
The four methodologies resulted in substantially different estimates of biomass-adjusted antimicrobial sales for use in US food animals. The 2018 estimates were the highest with the ESVAC methodology (314.7 mg of active antimicrobial ingredient/kg of animal biomass), followed by PHAC (191.5 mg/kg), FDA (127.6 mg/kg) and OIE (111.5 mg/kg). The animal weight parameters used in each methodology had the most impact on the biomass-adjusted sales estimates.
Conclusions
In regard to the estimation of the animal biomass, no methodology was found to be perfect; however, the FDA methodology had the best resolution in characterizing the US livestock biomass while the OIE methodology was best for biomass estimation for global monitoring of antimicrobial sales for use in food animals.
Introduction
Antimicrobial resistance is a global health crisis.1 While emergence and spread of antimicrobial resistance is a complex multicausal evolutionary phenomenon, antimicrobial use in food animals is a contributor to this crisis and a potential source of antimicrobial-resistant infections in humans.2,3 Current evidence shows that antimicrobial-resistant organisms can be transferred from food animals to humans through direct contact,4,5 the food chain6–9 and the environment,10,11 and shared between food animals and humans.12–16 The expanding human population is becoming more reliant on animals for food, which induces large-scale intensive farming operations and expands antimicrobial use in food animals. This adds to the ongoing problem of overuse and inappropriate use of antimicrobials in food animals and increases the health risks in humans from resistant organisms.17–19
In response to the global public health crisis of antimicrobial resistance, several countries have introduced restrictions on the use of antimicrobials in food animals. For example, use of veterinary antimicrobials for growth promotion was outlawed, prohibited or voluntarily withdrawn in the EU, Canada and the USA.20–22 Currently, antimicrobials are only approved for use in food animals to treat, control and prevent disease in these countries (and member countries of the EU).
In addition to the restrictions in the use of antimicrobials, monitoring antimicrobial use in animals also supports the fight against antimicrobial resistance.23 Monitoring antimicrobial use can be used to assess whether the regulations aimed at antimicrobial use are successful, help determine whether there is an excessive use of antimicrobials, guide future policies, provide a general understanding of veterinary antimicrobial use over time and, most importantly, help study the association between antimicrobial use and antimicrobial resistance.24–26 However, monitoring of the actual antimicrobial use in animals is challenging in terms of the required resources and infrastructure.27 Because antimicrobial sales data at the level of pharmaceutical companies and wholesalers are easier to obtain, they are frequently used for surveillance.17,19,28–30 Surveillance systems based on antimicrobial sales data have been considered essential for data retrieval on a global scale and inter-country comparison and as an initial step to gain knowledge on antimicrobial volumes in livestock husbandry or in the veterinary sector.30,31 However, it is important to emphasize that the sales data are not indicative of how antimicrobial drugs were actually used in animals (e.g. for what indications, doses or durations); consequently, management of antimicrobial resistance and antimicrobial stewardship efforts cannot be informed by the sales data alone.30,32 Sales data are affected by the composition of the population under study, and they do not reflect important aspects of the actual antimicrobial use, such as the availability of different drugs, with different potencies, for different diseases and species.28,33 Thus, the sales data can be used for assessing the amounts of antimicrobials introduced into the marketplace each year but there are limitations in what conclusions can be drawn if the data are used to make comparisons between countries, animal species, or antimicrobial drug classes.32
Organizations such as the FDA, European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) of the EMA, Public Health Agency of Canada (PHAC) and World Organisation for Animal Health (OIE) established surveillance systems for veterinary sales of antimicrobials.34–37 In general, these surveillance systems document national or regional antimicrobial sales by dividing the quantity of active ingredient (i.e. the chemical that prevents microbial growth or destroys microorganisms) sold by the size of the animal population potentially requiring the drugs (animal biomass). For example, it has been proposed that an animal biomass that is specific to the US domestic livestock populations and animal drug approvals would: (i) allow for the most appropriate representation of antimicrobial sales data relative to food animal biomass in the USA; (ii) adjust antimicrobial sales data to allow for additional trend analysis beyond the analysis of antimicrobial sales data alone; and (iii) allow the FDA to better interpret trends in antimicrobial sales data relative to US livestock populations.35 The above-mentioned organizations share the same goal: to monitor antimicrobial sales by controlling for animal demographics. However, the methodologies to achieve that goal differ in terms of underlying assumptions or in the estimation of the animal population that may be subject to antimicrobial treatment.26,28,38–41 It is not well understood whether these differences among methodologies could lead to meaningfully different estimates in animal biomass or conclusions, or even misinterpretations, and if any of the methodologies hold any advantages over each other.
In this study, we adjusted the annual US-specific antimicrobial sales for the biomass of the major food-producing species between 2016 and 2018 using the biomass methodologies established by the FDA, ESVAC, PHAC and OIE. Our objective was to review the four methodologies and compare their estimates. Additionally, we compared strengths and weaknesses of biomass estimation by the four methodologies in terms of the level of detail (resolution) accounted for in the US livestock biomass, ability to observe temporal trends in animal biomass within a country (to allow better interpretation of trends in antimicrobial sales in the country) and practicality in biomass estimation for comparing veterinary antimicrobial sales across countries.
Materials and methods
Data sources
To complete this study, three types of data were collected: (i) US-specific annual antimicrobial sales for a food-producing animal species, as well as (ii) annual population and (iii) annual average weight of the animal species that is under the risk of antimicrobial exposure. The sources, values and usage of the data we collected varied based on the application of each biomass methodology (i.e. FDA, ESVAC, PHAC and OIE). Details about the biomass methodologies were obtained from the publications and reports prepared by the FDA, ESVAC, PHAC and OIE and used for estimation of their respective biomass denominators (biomass denominators were calculated based on population and weight data for all methodologies).34–37,42–45
Data on US-specific antimicrobial sales were obtained from the FDA’s ‘2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals’, which contains information about the antimicrobials approved for use in US food-producing animals and reports the total quantity of those drugs that entered the market in 2018.46 In the FDA’s report, annual sales values are expressed in kilograms (kg), summarized by antimicrobial drug class and the major food-producing species (i.e. cattle, swine, chickens and turkeys). Antimicrobial drugs are further grouped by their medical importance (i.e. ‘medically important’ and ‘not-medically important’), based on their use in human medicine.47 Annual sales and distribution information for two previous reporting years (2016 and 2017) are also included in the 2018 report (Tables S1–S3, available as Supplementary data at JAC Online).
For all methodologies (FDA, ESVAC, PHAC and OIE), estimations of the annual livestock population and annual average weight relied mostly on three United States Department of Agriculture (USDA) sources: the National Agricultural Statistics Service (NASS),48–56 the Economic Research Service (ERS),57 and Foreign Agricultural Service (FAS).58 For data unavailable in these sources, e.g. for estimating annual average weights for animals living longer than a year, we used USDA Animal and Plant Health Inspection Service (APHIS) and non-USDA sources [i.e. Bovine Alliance on Management and Nutrition (BAMN) and Iowa State University Extension and Outreach].59–61 In the ESVAC and PHAC methodologies, annual average weights have previously been defined and standardized for each animal species to be used in their respective (member) countries. However, similar standardized weights do not exist for the USA; therefore, the standardized weights defined in the original ESVAC and PHAC protocols were used for US-specific calculations.34,36 For the OIE methodology, we used the datasets defined by the OIE to collect the US-specific population and weight data: the OIE World Animal Health Information System (WAHIS) and the United Nations Food and Agriculture Organization Statistics (FAOSTAT).62,63 Details about the data and sources for the four methodologies are provided in the Supplementary data grouped by methods (data for the FDA, ESVAC, PHAC and OIE are shown in Tables S4–S6, Tables S7 and S8, Tables S9 and S10, and Tables S11–S18, respectively). The biomass denominators of the four methodologies were reviewed and compared, and their differences and similarities were summarized graphically (Figure 1).
Figure 1.
Comparison of biomass denominators in the FDA, ESVAC, PHAC and OIE methodologies. ‘Livingstock’ are defined here as animals raised longer than one calendar year, which in the biomass methodologies applies to beef cattle, dairy cattle and swine.
Biomass denominators
A biomass denominator is typically defined as the product of the annual population size of a given food-producing animal species and the annual average weight of an individual animal of the species. It represents the annual weight of the animal population potentially being treated with antimicrobial drugs. The US-specific animal biomasses for the major food-producing animal species were calculated by the FDA, ESVAC, PHAC and OIE methodologies for 2016, 2017 and 2018 (Tables S4–S18). The methodologies and their application to the US data are explained in the following paragraphs.
The biomass denominator of the FDA methodology, called the ‘Target Animal Biomass’ (TAB), is defined as the product of the annual population size and ‘average weight at slaughter’ for each animal species (Equation 1).
| (1) |
The ESVAC and PHAC denominators (called the ‘Population Correction Unit’; PCUEU and PCUCAN, respectively) were calculated by multiplying the annual population size of an animal species by the average animal weight at the time of antimicrobial treatment, referred to as the ‘standard weight at treatment’ (Equation 2).34,36 Both methodologies use standard, pre-defined values for animal weight, with ESVAC using their values representing the characteristics of EU and European Economic Area (EEA) countries, and with PHAC using their values to represent characteristics of the animal production in Canada. Another difference between the two methodologies is that the PHAC estimation includes biomass of beef cows raised longer than one calendar year (animals raised longer that one calendar year are thereafter referred to as ‘livingstock’ and are defined below), which is not included in the ESVAC method, because the population of non-dairy livingstock cows was deemed negligible in EU and EEA countries at the time the ESVAC method was established. Livingstock beef cows are included in PCUCAN, as they are considered to be a significant cattle subpopulation in Canada.36
| (2) |
The FDA, ESVAC and PHAC methodologies calculate animal biomass as a function of slaughtered and livingstock animals, as well as imported and exported animals (Equation 3):
| (3) |
where can be either TAB, PCUEU or PCUCAN and is either Slaughtered, Livingstock, Export or Import. For a given species, biomass of all animals raised less than a year was accounted for by , while was used to account for the annual biomass estimate of animals that are raised longer than one calendar year in the USA. These animals include livingstock beef cattle (i.e. all beef cows that have calved—again, these are not included in PCUEU, but included in TAB and PCUCAN), livingstock dairy cattle (i.e. all dairy cows that have calved—these are included in TAB, PCUEU and PCUCAN) and livingstock swine (i.e. all hogs and pigs kept for breeding, including boars—these are included in TAB, PCUEU and PCUCAN). For livingstock beef and dairy cows, the January Cattle Inventory report from USDA NASS was used as a source of information about the animal population size as it provides the national estimates for the previous year in the USA.50,51 For livingstock swine, the December Hogs and Pigs Inventory from USDA NASS was used for national estimates of the population size of the current year.52–54 was not applicable to chickens and turkeys in these methodologies, since these animals are typically slaughtered within a year from hatching. was added to the total biomass denominator and represented the animals exported from the USA, as exported animals are according to the FDA, ESVAC and PHAC assumed to be treated with antimicrobials marketed in the USA. According to these methodologies, it was also assumed that the animals are treated with antimicrobials prior to being imported into the USA; therefore, , which represented the biomass of animals imported into the USA, was subtracted from the total biomass denominator.
The OIE denominator, called the ‘Animal Biomass’, was calculated as the total weight of the food animal species present in the USA in a year. Contrary to the use of data on live weights of animals in the FDA, ESVAC and PHAC denominators (i.e. weight at slaughter or weight at treatment), the OIE uses data on carcass weights along with the number of slaughtered animals provided by FAOSTAT. Accordingly, first, an average carcass weight was calculated for each species.45 Average carcass weight was then converted to the ‘average weight at slaughter’ (which is in the OIE methodology referred to as the ‘live weight’) by dividing it with a conversion coefficient specific to each species (Equation 4):64
| (4) |
Next, for chickens and turkeys, the animal biomass was calculated by multiplying the calculated weight at slaughter by the number of slaughtered animals, while cattle and swine biomasses were calculated by multiplying weight at slaughter by the census population provided by WAHIS Interface (Equation 5):
| (5) |
In the OIE methodology, additional factors are used for swine and cattle to better represent their animal biomasses. Specifically, the cattle biomass includes factors for age and production classes (calves, younglings and adult cattle). For the swine biomass, an additional swine population was included by applying a factor to the census population to account for sows raised for more than a year for breeding purpose. In the OIE biomass methodology, imported and exported animals are accounted only for cattle but not for other major food species.
All biomass estimates were expressed in kg (i.e. 1 kg = 1 TAB = 1 PCUEU = 1 PCUCAN = 1 Animal Biomass). For a methodology, the total biomass was calculated by pooling the species-specific biomass estimates including the biomasses of cattle, swine, chickens and turkeys.
Biomass-adjusted sales
Unadjusted antimicrobial sales (i.e. antimicrobial sales for use in cattle, swine, chickens and turkeys reported by the FDA) for a year were adjusted by the total animal biomass (including the biomass of cattle, swine, chickens and turkeys) estimated by the FDA, ESVAC, PHAC and OIE methodologies. Similarly, species-specific annual antimicrobial sales (i.e. unadjusted antimicrobial sales for use in each of the major food-producing species) were adjusted by the corresponding species-specific biomass denominator for the year. Biomass-adjusted antimicrobial sales were expressed in milligrams (mg) of an active antimicrobial ingredient per kg of animal biomass. For each methodology, antimicrobial sales were grouped by animal species, medical importance and antimicrobial drug class, and adjusted by the methodology specific animal biomass estimate. When presenting results, the availability of the FDA’s US-specific national (unadjusted) antimicrobial sales data and existing approaches to the presentation of results for respective methodologies done by the FDA,35 PHAC,36 ESVAC34 and/or OIE37 were followed and then the same approach to the presentation of results was applied across all methodologies to assure consistency and comparability among methods. The same calculations were conducted for years 2016, 2017 and 2018.
Data analysis
Percent change was used as a metric to compare annual trends between sales and biomass data, using the following equation (Equation 6):
| (6) |
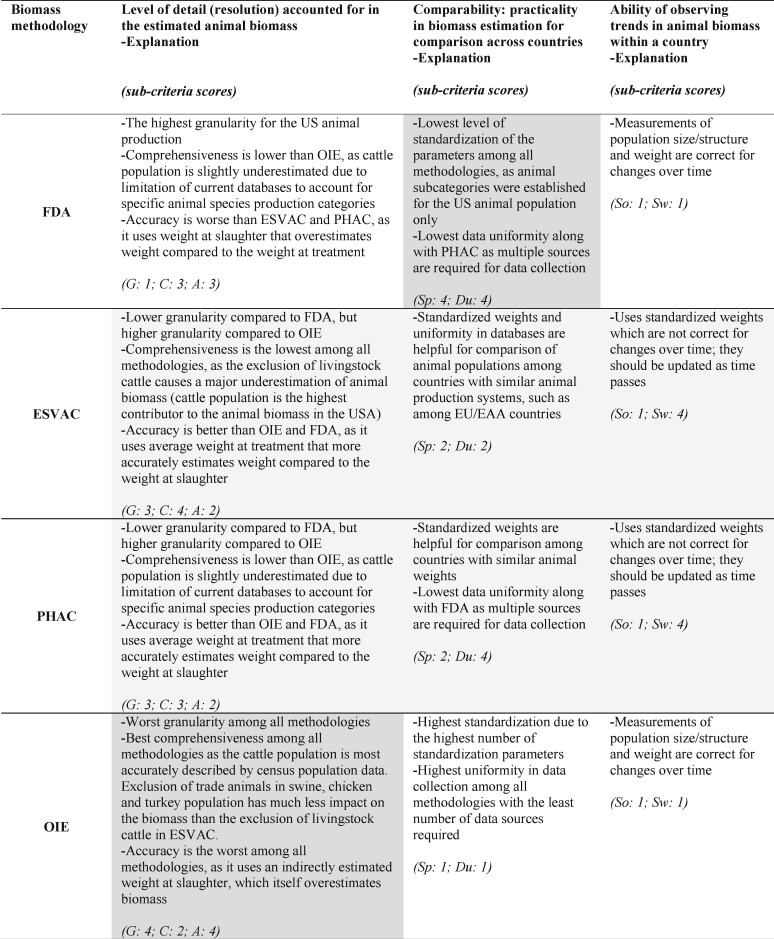
Biomass denominators of the four methodologies were qualitatively compared according to three criteria: (i) the level of detail (resolution) accounted for in the estimated US livestock biomass; (ii) ability to track temporal trends in animal biomass in a country (for interpretation of trends in antimicrobial sales in the country); and (iii) practicality in biomass estimation for comparing antimicrobial sales among different countries. These comparisons were aided by the application of seven sub-criteria adapted from Collineau et al.26 (2017), and described in Table 1. The sub-criteria were Granularity (G), Comprehensiveness (C), Accuracy (A), Standardized parameters (Sp), Data uniformity (Du), Stability in population (So) and Stability in weights (Sw); each methodology was scored 1 (best) to 4 (worst) for the seven sub-criteria. The sub-criteria G, C and A were used for evaluating the biomass denominators in terms of the level of detail accounted for in the estimated US animal biomass; sub-criteria Sp and Du were used for evaluating the practicality in estimation of animal biomass for comparing adjusted antimicrobial sales across countries; and sub-criteria So and Sw were used to evaluate the biomass denominators’ ability to track temporal trends in animal biomass in a country.
Table 1.
Criteria and sub-criteria applied to evaluate the utility of biomass denominators in the FDA, ESVAC, PHAC and OIE methodologies
| Criteria | Sub-criteria | Notation | Definition |
|---|---|---|---|
| Level of detail (resolution) accounted for in the estimated animal biomass (Which biomass estimate provides the highest resolution in the estimated US livestock biomass?) | Granularity | G | Number of specific animal production categories considered for each species |
| Comprehensiveness | C | Capacity to collect population data for all animals of a species | |
| Accuracy | A | Use of actual treatment weight data per species | |
|
| |||
| Comparability: practicality in biomass estimation for comparison across countries (Can we estimate animal biomass to compare sales across countries?) | Standardized parameters | Sp | Use of standardized values for estimating animal biomass |
| Data uniformity | Du | Availability of centralized sources for data collection | |
|
| |||
| Ability of observing trends in animal biomass within a country (Can we observe trends over time?) | Stability in population | So | Size and structure of population at risk of being treated correct for changes over time |
| Stability in weights | Sw | Weight of animals at risk of being treated correct for changes over time | |
Data sources and assumptions underlying the four methodologies are detailed in the Supplementary data. Excel spreadsheets were used for organization of data inputs and calculations; the spreadsheets are available at https://github.com/IvanekLab/Biomass-Methodologies-USA.
Results
Biomass-adjusted sales by the FDA methodology
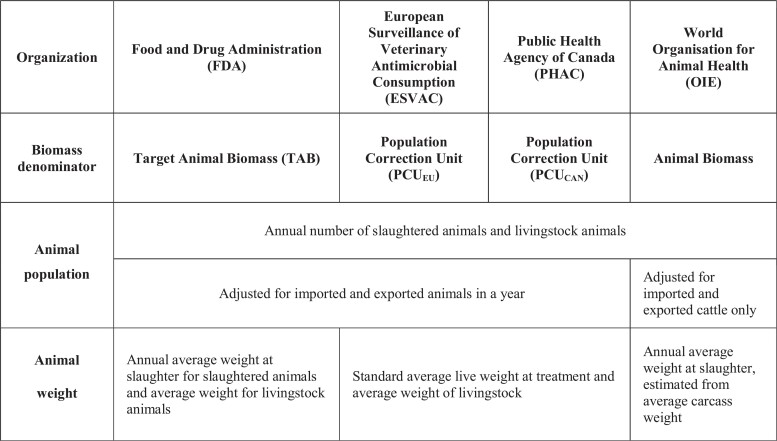
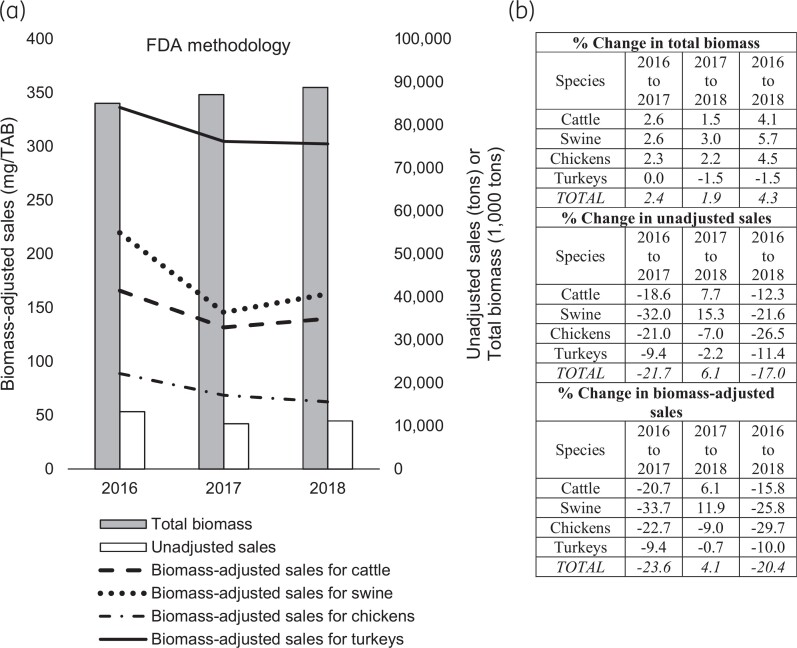
Between 2016 and 2018, the FDA’s TAB for the major food-producing animal species (cattle, swine, chickens and turkeys) grew by 4.3%, from 85.0 billion kg to 88.7 billion kg. At the species level, swine, chickens and cattle biomasses increased by 5.7%, 4.5% and 4.1%, respectively, while turkey biomass decreased by 1.5% (Figure 2). During the same period, unadjusted antimicrobial sales for the major food-producing animal species decreased by 17.0%, from 13.6 million kg in 2016 to 11.3 million kg in 2018, as reported by the FDA (Tables S1–S3; unadjusted antimicrobial sales represent the sum of medically important and not-medically important antimicrobial sales). The reduction in unadjusted antimicrobial sales reflects a major drop of 21.7% in antimicrobial sales observed in 2017 following the full implementation of Guidance For Industry (GFI) #209 and #213 and the changes to the Veterinary Feed Directive (VFD), which moved to eliminate the use of medically important drugs for production purposes (i.e. growth promotion and feed efficiency) while allowing their administration for prevention, control and treatment of diseases under the supervision of licensed veterinarians only.21,65,66 A year after the implementation of the GFIs and the changes to the VFD, in 2018, an increase of 6.1% was observed in the unadjusted antimicrobial sales compared with 2017. As the GFIs and the VFD affected medically important antimicrobial sales, a much larger decrease (33.9%) was observed in the unadjusted sales of medically important antimicrobials in 2017 compared with 2016 (Figure 3). However, the decrease was short lasting, as about a 9.3% increase in the unadjusted sales was reported between 2017 and 2018.
Figure 2.
Total sales of veterinary antimicrobials between 2016 and 2018. (a) Bars indicate the unadjusted sales and the total biomass. Lines indicate the biomass-adjusted sales by animal species. The FDA’s biomass adjustment methodology was used to estimate the total biomass and biomass-adjusted sales. The unit of TAB is kg. (b) Percent change in biomass-adjusted antimicrobial sales, total animal biomass and unadjusted antimicrobial sales by year and species. Data on unadjusted sales are from the FDA’s ‘2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals’.46
Figure 3.
Sales of medically important veterinary antimicrobials between 2016 and 2018. (a) Bars indicate the unadjusted medically important antimicrobial sales and the total biomass. Lines indicate the biomass-adjusted medically important antimicrobial sales by animal species. The FDA’s biomass adjustment methodology was used to estimate the total biomass and biomass-adjusted medically important antimicrobial sales. The unit of TAB is kg. (b) Percent change in biomass-adjusted medically important antimicrobial sales, total animal biomass and unadjusted medically important antimicrobial sales by year and species. Data on unadjusted sales are from the FDA’s ‘2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals’.46
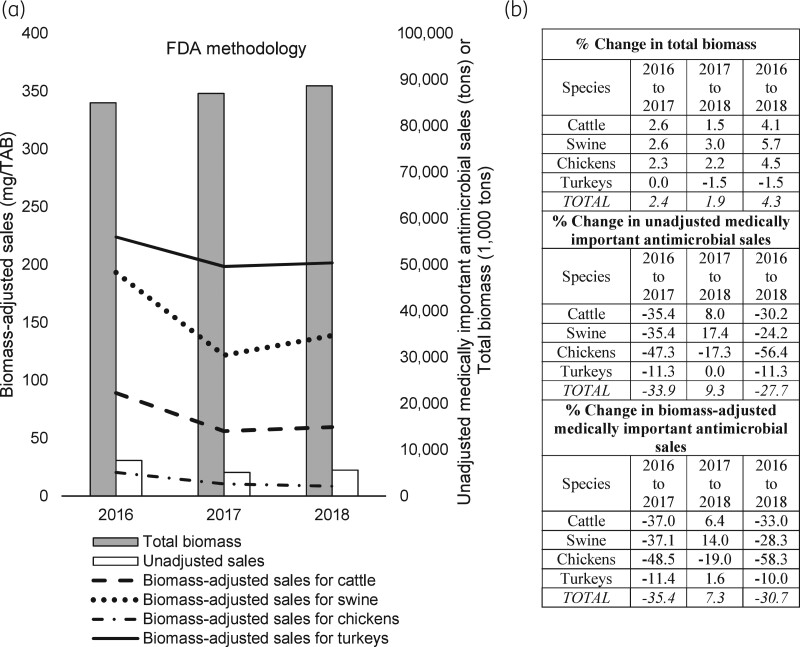
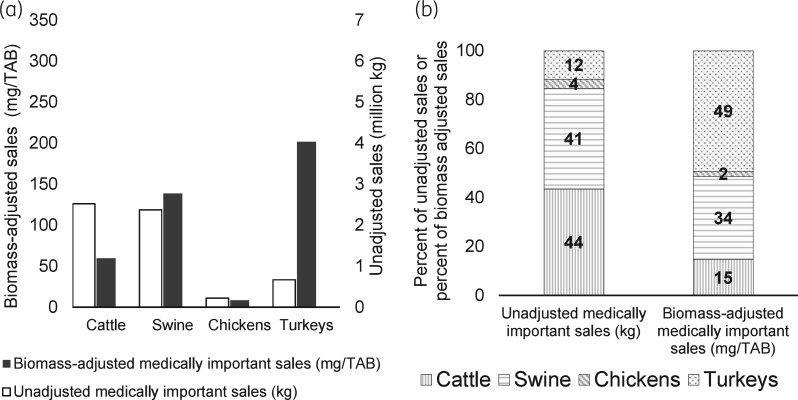
Of 11.3 million kg of unadjusted antimicrobial sales for use in major food-producing animal species in the USA in 2018, about half were for use in cattle (52%), and the remaining antimicrobials were sold for swine (25%), chickens (14%) and turkeys (9%) (Table S3).46 Using the FDA’s biomass methodology, the species-specific TAB in 2018 was 42.2 billion kg for cattle, 26.0 billion kg for chickens, 17.1 billion kg for swine and 3.3 billion kg for turkeys (Table S6). Using unadjusted antimicrobial sales data and the biomass estimates, the highest biomass-adjusted sales were estimated for turkeys (302.3 mg/TAB), followed by swine (162.9 mg/TAB), cattle (139.7 mg/TAB) and chickens (62.4 mg/TAB) (Table S19). Similar to the species-specific estimates for the FDA methodology,46 Figure 4 shows differences in species-specific unadjusted and biomass-adjusted sales in 2018. For example, the highest antimicrobial sales per kg of animal biomass were in turkeys, although the unadjusted sales were the lowest for turkeys. Unadjusted antimicrobial sales for cattle were the highest; however, after the biomass adjustment, sales per kg of animal biomass for cattle were behind those for turkeys and swine.
Figure 4.
Total sales of veterinary antimicrobials in 2018 by animal species. (a) Unadjusted sales reported by the FDA and biomass-adjusted sales estimated by the FDA’s biomass adjustment methodology. The unit of TAB is kg. (b) Percent of unadjusted sales and biomass-adjusted sales by species. Data on unadjusted sales are from the FDA’s ‘2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals’.46
Figure 5 shows the species-specific unadjusted and biomass-adjusted medically important antimicrobial sales for 2018 estimated using the FDA methodology (Tables S1 and S20). Of the 5.8 million kg of medically important antimicrobials sales (unadjusted), cattle (44%) and swine (41%) accounted for the most, while sales for turkeys (12%) and chickens (4%) were lower. Figure 5 also illustrates that biomass-adjusted sales of medically important antimicrobials were the highest for turkeys (201.5 mg/TAB) and swine (138.7 mg/TAB), followed by cattle (59.7 mg/TAB) and chickens (8.5 mg/TAB). Thus, ranking of medically important antimicrobial sales for cattle and turkeys changed after adjustment by biomass. For both the unadjusted and biomass-adjusted sales, the second highest sales were for swine and the lowest sales were for chickens. Unadjusted and biomass-adjusted sales for not-medically important antimicrobials are shown in Figure S1.47 Again, the biomass-adjusted sales were the highest in turkeys for not-medically important antimicrobials.
Figure 5.
Sales of medically important veterinary antimicrobials in 2018 by animal species. (a) Unadjusted medically important sales reported by the FDA and biomass-adjusted medically important sales estimated by the FDA’s biomass adjustment methodology. The unit of TAB is kg. (b) Percent of unadjusted medically important sales and biomass-adjusted medically important sales by species. Data on unadjusted sales are from the FDA’s ‘2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals’.46
The FDA’s biomass adjustment methodology was also applied to sales of certain classes of antimicrobials when details of species- and drug-specific sales were available in the FDA’s annual report.46 Thus, biomass adjustment for the major food-producing animal species (cattle, swine, chickens and turkeys) was conducted for aminoglycosides, macrolides and tetracyclines (Figures S2, S3 and S4). According to the FDA report, tetracyclines, which represent the largest volume of sales in 2018 (3.9 million kg) following ionophores, were intended for use mostly in swine (1.9 million kg, 48% of the total tetracycline [unadjusted] sales). When the sales were adjusted for animal biomass, the tetracycline sales per kg of animal biomass were also the highest for swine (111.2 mg/TAB). For cephalosporin, lincosamide, penicillin and sulfa sales, the biomass adjustment could be conducted for certain animal species only (Figures S5–S8).
Regarding temporal trends, biomass-adjusted sales were reduced by 20.4% for the total antimicrobial sales for the major food-producing species (127.6 mg/TAB) and 30.7% for medically important antimicrobial sales (65.3 mg/TAB) in 2018, compared with 2016. Figure 2 shows the change in the unadjusted antimicrobial sales, animal biomass and biomass-adjusted sales for the major food-producing animals (cattle, swine, chickens and turkeys) between 2016 and 2018. Figure 3 shows the same data for medically important antimicrobials.
Comparison of the FDA, ESVAC, PHAC and OIE methodologies
Biomass estimates by the FDA, ESVAC, PHAC and OIE methodologies varied due to the differences in how they estimate animal population size and parametrize animal weights. Figure 1 summarizes the main differences among the methodologies. In general, animal population size is estimated similarly for all four methodologies: they all include the number of slaughtered and livingstock animals in a year, and all, except for the OIE methodology, account for the number of traded animals for all species (OIE accounts only for cattle that are imported/exported). Significant differences were identified among animal weight parameters used by the four methodologies. In the OIE and FDA methodologies, weight parameters used for animal species include year-specific values that account for year-to-year changes in animal weights; however, standard average weights are used for all animals in the ESVAC and PHAC methodologies (i.e. the same weight parameters are used over time), thus annual changes in weights were not able to be observed. The OIE methodology starts with average carcass weights and converts them to average weights at slaughter for all animals. On the other hand, weight parameter of the FDA methodology (average weight at slaughter) defines the weight of slaughtered and traded animals using weights of animals at slaughter reported in the USDA databases. Living animal weights in the FDA methodology were adopted from non-USDA sources to calculate the biomass for livingstock animals, since ‘average weight at slaughter’ was not available in USDA-based sources (see the Supplementary data for details of the data sources and weight parameters for each animal category). Compared with FDA and OIE, the ESVAC and PHAC methodologies use weight at treatment for all animals.
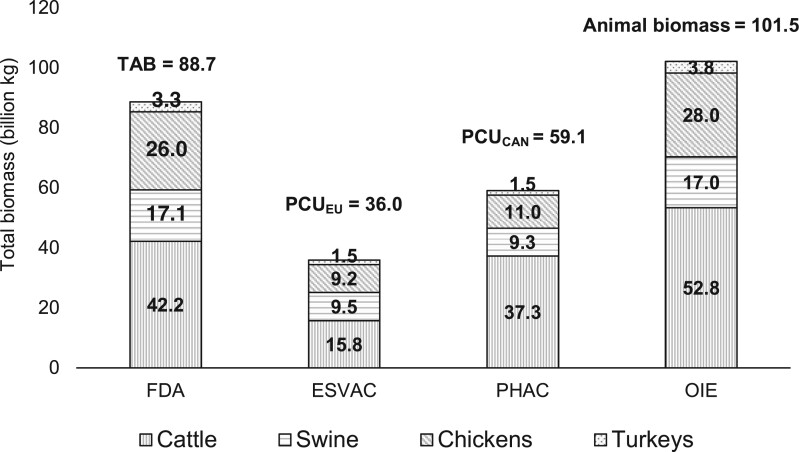
The 2018 US-specific estimates of the animal biomass by the FDA, ESVAC, PHAC and OIE methodologies are shown in Figure 6. Accordingly, the total estimated biomass for the major food-producing animal species was the lowest when the ESVAC methodology was used (36.0 billion kg). One of the reasons for that was that the FDA, PHAC and OIE methodologies account for livingstock beef cows, which are not included in the biomass of the ESVAC methodology. As a result, 31.8 million livingstock beef cows slaughtered in 2018 were not included in the biomass estimation by the ESVAC methodology. As a result, the cattle-specific biomass estimate was 15.8 billion kg by the ESVAC methodology, while the PHAC, FDA and OIE estimates were 37.3, 42.2 and 52.8 billion kg, respectively. If the livingstock beef cattle were included in the ESVAC methodology, the cattle biomass would increase by 13.5 billion kg, resulting in 29.3 billion kg for cattle-specific PCUEU (under the assumption that the weight at treatment for livingstock and slaughtered beef cows is the same).
Figure 6.
Total animal biomass in the USA in 2018 estimated by the FDA, ESVAC, PHAC and OIE methodologies
In general, biomass estimates by the OIE (101.5 billion kg) and FDA methodologies (88.7 billion kg) were higher than by the ESVAC (36.0 billion kg) and PHAC (59.1 billion kg) methodologies. That is because the weights at slaughter were used for the FDA and OIE estimations, which were almost always higher than the weight at treatment that was used in the ESVAC and PHAC approaches (Figure 6). Table 2 shows weights used for slaughtered animals for each of the four methodologies, since slaughtered animals were the main contributor to the animal population in the USA (i.e. considering the major food-producing animals only: cattle, swine, chickens and turkeys), and they largely explain the difference in biomass estimates among the methodologies. For example, the estimated cattle-specific biomass was the highest by the OIE methodology, because OIE used the highest weight for slaughtered (adult) cattle (772 kg). Weights used for slaughtered swine and turkeys in the ESVAC and PHAC were about half of the weights used by the FDA and OIE; therefore, the estimated biomasses for swine and turkeys were about twice as much for the FDA and OIE than for the ESVAC and PHAC methodologies. Similarly, the weight of a slaughtered chicken was about three times more in the OIE and FDA compared with the ESVAC and PHAC methodologies, which resulted in biomass estimates for the FDA and OIE that were about three times of the ESVAC's and PHAC’s (Figure 6).
Table 2.
Comparison of animal weight parameters among the FDA, ESVAC, PHAC and OIE methodologies for 2018 biomass estimations of slaughtered animals
| Species | FDA (average weight at slaughter, kg) | ESVAC (standard weight at treatment, kg) | PHAC (standard weight at treatment, kg) | OIE (average weight at slaughter, kg) |
|---|---|---|---|---|
| Slaughtered (adult) cattle | 612 | 425 | 600 | 772 |
| Slaughtered swine | 128 | 65 | 65 | 123 |
| Slaughtered chickens | 2.8 | 1 | 1.2 | 3.1 |
| Slaughtered turkeys | 14.1 | 6.5 | 6.5 | 16.1 |
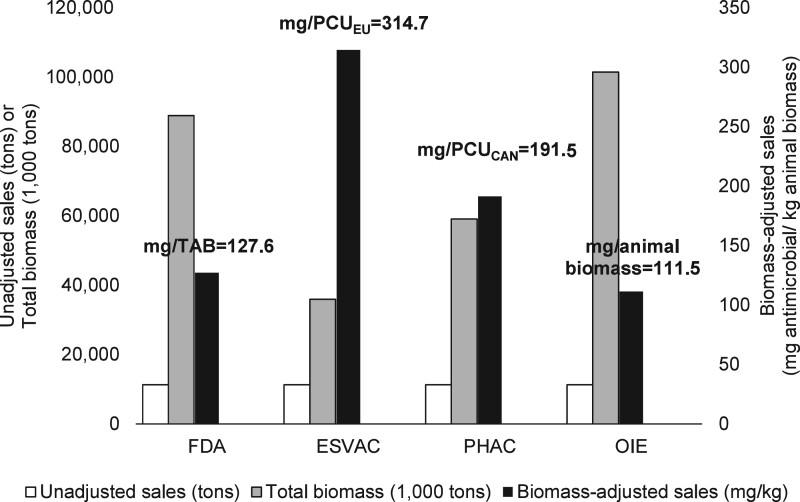
The differences among the US-specific biomass-adjusted sales estimated by the FDA, ESVAC, PHAC and OIE methodologies for 2018 are illustrated in Figure 7. Because the estimations were conducted for the USA, the unadjusted antimicrobial sales were the same for all four methods, i.e. 11.3 million kg that was sold for use in cattle, swine, chickens and turkeys in 2018, as reported by the FDA. Because the biomass denominators for the major food-producing species were the highest for the OIE (101.5 billion kg) and FDA (88.7 billion kg) methodologies, the corresponding biomass-adjusted sales by these methodologies were the lowest (OIE = 111.5 mg antimicrobial/kg animal and FDA = 127.6 mg/kg). The highest adjusted sales estimate was for the ESVAC methodology (314.7 mg/kg), which was followed by the PHAC (191.5 mg/kg). Figure 8 shows the results of the qualitative comparison among the biomass denominators of the four methodologies as to whether they are able to account for population details in the estimation of the US animal biomass, allow observing trends in animal biomass over time and are practical for biomass estimation for comparison of adjusted sales among different countries; scores for underlying sub-criteria and explanations of the scores are shown in Table S21. Accordingly, the FDA’s biomass estimate was judged as the one providing the highest resolution (level of detail) in the estimated biomass of the US livestock, while the OIE’s biomass estimate was considered the most suitable for cross-country comparisons of antimicrobial sales (comparability). Biomass estimates of the FDA and OIE methodologies were both deemed the most useful for observing trends in biomass estimates within a country over time. Biomass-adjusted medically important antimicrobial sales estimated by each methodology for 2018 are illustrated in Figure S9. Trends of the estimates between 2016 and 2018 are depicted for each methodology in Table S20.
Figure 7.
Unadjusted antimicrobial sales, total biomass and biomass-adjusted sales estimated by the FDA, ESVAC, PHAC and OIE methodologies. The unit of TAB, PCUEU, PCUCAN and Animal biomass is kg. Data on unadjusted sales are from the FDA’s ‘2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals’.46
Figure 8.
Comparison of the FDA, ESVAC, PHAC and OIE biomass methodologies by three criteria. The criteria are the level of detail (resolution) accounted for in the estimated US livestock biomass, comparability in terms of practicality in estimating animal biomass for comparison of antimicrobial sales across countries and the ability to observe temporal trends in animal biomass. Sub-criteria for each criterion were scored 1 (best) to 4 (worst) and scores are indicated in parentheses. Definitions of sub-criteria are provided in Table 1. The overall level of suitability of a methodology, i.e. good, neutral or bad, for a criterion is indicated by white, light grey and dark grey, respectively.
Discussion
In this study, we reviewed and compared the FDA, ESVAC, PHAC and OIE biomass adjustment methodologies by using them in the estimation of biomass-adjusted antimicrobial sales for cattle, swine, chickens and turkeys between 2016 and 2018 in the USA.
We observed an overall reduction in the total antimicrobial sales (including unadjusted and biomass-adjusted sales) in 2017, which was followed with an increase in 2018. The decline in 2017 is attributed to the implementation of the GFIs and the changes to the VFD, which resulted in considerable reductions in sales of medically important antimicrobials. A similar trend in antimicrobial sales was observed in many European countries during the period when antimicrobials used for growth promotion were phased out (late 1990s and early 2000s), where an increase was observed in the national sales of veterinary antimicrobials for therapeutic purposes.67–69 Specifically, unadjusted or total sales of medically important antimicrobials in Europe were increased for use in swine and cattle populations.70,71 Interestingly, a similar increasing trend in the unadjusted sales of medically important antimicrobials was observed for the US swine and cattle populations in 2018, which is the year following the implementation of the GFIs and the changes to the VFD (the percent change in unadjusted sales of medically important antimicrobials was +17.4% for swine and +8.0% for cattle in 2018 compared with 2017; Figure 3b). It would be of interest to determine in future research whether this increase in the US antimicrobial sales (unadjusted) may have been at least partially explained by an increased disease burden requiring antimicrobial therapy, similar to the European experience.
In early 2000s and after, following the ban of using antimicrobials for growth promotion, the animal production levels in several European countries remained the same, or they increased (for chickens, turkeys and cattle),68,72,73 though there was a temporary decrease in the weaner swine population in Denmark and Sweden.73,74 In contrast, the US population of the major animal species, except turkeys, continued to increase following the implementation of the GFIs and the changes to the VFD. The population of turkeys decreased during that time; however, this trend seems to be independent from the GFIs and the VFD, as the population had been decreasing already in the previous years (Table S4).
It has been recognized that antimicrobial sales data can be used to assess trends and broad shifts in the amounts of antimicrobials sold for use in animals annually.32 In our study, between 2016 and 2018, the cattle-specific unadjusted total and medically important antimicrobial sales were the highest compared with sales of remaining major food animals; however, when the biomass adjustment was applied, the highest sales of total and medically important antimicrobials were for turkeys. As demonstrated, biomass-adjusted sales and unadjusted sales led to different types of insights into antimicrobial sales for use in the US food animal production. Similar to what has been suggested by the Pew Charitable Trusts (2019), compared with the FDA’s (unadjusted) national sales data, the biomass-adjusted sales accounted for the variations in the size and composition of the animal populations, and consequently provided a different view into the sales data that better reflects the populations under study.25 However, whether antimicrobial sales data are adjusted by biomass or unadjusted, they provide no information about the amount of antimicrobials actually used or whether the use was judicious. For example, in our study, the biomass-adjusted sales estimates were the highest for turkeys (across all four methodologies). However, in reality, much of the antimicrobials sold for use in turkeys are administered to control diseases with high incidence rates75–78 due to lack of approved efficacious drugs or use of antimicrobials with low potency (i.e. use of high molecular weight antimicrobials or use of higher grams administered per regimen),75,76,78,79 thus inflating the volume of the sales. This demonstrates how using the antimicrobial sales data alone could be misleading in interpreting the actual antimicrobial use in a population and should be discouraged.
The choice of animal weight parameters in the calculation of the biomass denominator has a profound effect on the adjusted sales estimates. The weight parameters used in all methodologies were limited in representing the actual animal weights, because they are not accounting for the fact that different diseases affect an animal species at different ages, and different antibiotics are used for these different diseases. In our study, biomass-adjusted sales estimated by the OIE and FDA methodologies were lower than the estimates by the ESVAC and PHAC. That is mainly because the OIE and FDA methodologies use weight at slaughter parameters, which were almost always higher than the weight at treatment used by the PHAC and ESVAC methodologies.34–37 Using weight at slaughter for an animal overestimates the weight of the total animal population that is exposed to antimicrobials, as antimicrobial treatments are most likely administrated at an earlier time in the animal’s life.25 Therefore, weight of an animal at the time it would most likely be treated with antimicrobials, or weight at treatment used by the ESVAC and PHAC methodologies, is likely more appropriate for estimating the total animal biomass. From this perspective, the ESVAC’s and PHAC’s choice for weight at treatment seems advantageous compared with the OIE’s and FDA’s weights; however, it is important to emphasize that the ESVAC's and PHAC's weights represent animal population in their respective countries, and they could not be adopted by the FDA methodology to represent the US animal population due to the differences in the animal agriculture industry between the countries. In addition, the use of the exact same standard values for weights year-to-year does not account for the potential variations in weight for an animal population over time or regionally, which might be affected by many factors such as differences in husbandry practices80,81 or even global disruption of food systems such as during the Corona Virus Disease 2019 (COVID-19) pandemic.82 In contrast, FDA’s and OIE’s weights at slaughter are estimated annually, which allows one to observe changes in animal weights for any potential reason. Overall, it might be a valuable future direction for the FDA methodology to use the best of both worlds—a combination of the weight systems, which would be accurate for estimating the size of animal population that is under the risk of antimicrobial exposure, specific for representing the US animal population and flexible for observing annual weight changes across animal populations.
The criteria and sub-criteria used for evaluating the biomass denominators of the four methodologies identified several important differences (Figure 8). Accordingly, the biomass denominator of the OIE methodology was considered the top candidate for estimating biomass to compare veterinary antimicrobial sales across countries. The biomass denominator of the FDA methodology was considered the most suitable for providing the highest resolution in estimation of the US livestock biomass. Finally, denominators of the FDA and OIE methodologies were tied for their ability to observe trends in animal biomass within a country over time. The details of the evaluations are provided in the following sections (and also shown in Table S21).
We evaluated the methodologies for their ability to estimate the animal biomass for comparison of antimicrobial sales among different countries (Figure 8), as such comparisons are used for gaining insights into the volume of antimicrobials available for use in animals globally and to contribute to the global fight against antimicrobial resistance.19,29,31,37,39,83 For example, such comparisons among countries have been done by the OIE and PHAC methodologies.36,37 An implicit assumption, necessary when comparing antimicrobial sales across countries, is that approval of drugs in compared countries is the same. With this assumption, in our study, the biomass denominator of the OIE methodology was judged as the most suitable among the four methodologies for biomass estimation to support comparison of biomass-adjusted sales between countries. The OIE methodology aims to contribute to the control of antimicrobial resistance globally; therefore, its methodology was designed to collect and analyse data on antimicrobials intended for use in animals and animal production from all countries.37 Accordingly, the OIE’s methodology is highly standardized for estimating animal biomass and biomass-adjusted sales, which makes it relatively easy to compare antimicrobial sales among countries globally (best in sub-criterion Sp, Table S21).37 In addition, the methodology requires only two centralized databases for data collection to estimate the animal biomass (FAOSTAT and WAHIS); these databases are globally available for all countries (best in sub-criterion Du, Table S21).62,63 From these databases, only four types of data were required to estimate the country specific biomass of cattle, swine, chickens and turkeys: (i) annual population (reported by FAOSTAT); (ii) total weight of slaughtered animals (reported by FAOSTAT); (iii) annual census population of cattle and swine (reported by WAHIS); and (iv) traded cattle population (reported by FAOSTAT). After data collection, biomass is estimated using coefficients that are standardized for each species. Overall, the OIE methodology has characteristics that are essential for cross-country comparisons: uniformity in the available type of data and standardization of the parameter use.24,26,40 In comparison, the ESVAC and PHAC methodologies can still be useful for comparing animal biomass and adjusted sales between countries with similar animal production systems owing to the use of standard average weights in estimated biomass denominators. However, cross-country comparisons by these methodologies might result in misinterpretations when the comparison is done with countries with different animal demographics, whose animal population does not fit to the categories and/or parameter values established by the ESVAC and PHAC.39 This was demonstrated in our study when the ESVAC methodology was used to describe the US-specific animal population: exclusion of livingstock beef cows as a separate category in PCUEU resulted in an underestimation of the size of the cattle population in the USA, by excluding 43.4% of the total cattle population in 2018. Although the denominators in both methodologies have similar levels of standardization in weight parameters (sub-criterion Sp), the data uniformity (sub-criterion Du) for the PHAC denominator is lower compared with the ESVAC denominator, as the PHAC denominator requires multiple databases to collect information while the ESVAC uses only two international databases that would apply to all the EU/EAA countries (Eurostat and TRACES).34,37 Among all the four methodologies, the biomass denominator of the FDA methodology is the least suitable for tracking biomass of food animals globally, as it was established to capture characteristics of the US animal population only; therefore, it has the least number of standard parameters to estimate the US-specific animal biomass. In addition, similar to PHAC, the FDA methodology depends on multiple databases to collect information. Due to these characteristics, the denominator of the FDA methodology may not represent the animal population in another country and could cause problems in application and interpretation when used for biomass adjustment of other countries’ veterinary antimicrobial sales.
We assessed the biomass denominator of the four methodologies regarding the level of detail they accounted for in the biomass estimates for the USA, which is an important aspect considering that a higher resolution provides a finer look into the overall livestock biomass in a country that would allow better interpretation of trends in antimicrobial sales in the country (Figure 8).84 In general, all biomass denominators had limitations considering evaluation sub-criteria Granularity (G), Comprehensiveness (C) and Accuracy (A). Nevertheless, the denominator of the FDA methodology produced the biomass estimates with the highest level of detail (resolution), due to its inherent specificity in representing the US animal production. Particularly, the FDA methodology allows for categorization of animals specific to the US with the best granularity among all methodologies (e.g. cattle imports for immediate slaughter, cattle and calves imported for feeding), which strengthens the methodology for the US biomass estimation (best in sub-criterion G, Table S21). Considering all sub-criteria for resolution in the biomass estimate, the ESVAC and PHAC methodologies are not as useful for determining the adjusted sales for the US animal production system, even though the ESVAC’s and PHAC’s weight parameters are more realistic compared with the FDA’s weight parameters (sub-criterion A). Between the two methodologies, the ESVAC would produce biomass estimates with an even lower resolution, since it does not account for livingstock beef cattle and thus it lacks comprehensiveness, as mentioned earlier (worst in sub-criterion C, Table S21). The denominator of the OIE methodology was considered the least useful for estimating the US animal biomass, as the methodology results in crude estimates of biomass mainly due to low granularity in animal sub-categories and the use of standardized elements in calculations (worst in sub-criteria G and A, Table S15 and Table S21). It is important to note that the OIE methodology has better comprehensiveness than all other methodologies as census population data are used for all animal species, which allows estimation of the population of a species better in a point of time, unlike the other biomass methodologies that estimate animal population by summing animal sub-groups annually. Comprehensiveness of the OIE denominator is better than all other denominators, even though animal trade data are accounted for cattle only since trade animals are minor components of the US animal biomass calculation.
According to our sub-criteria Stability in population (So) and Stability in weights (Sw), the biomass denominators of the FDA and OIE methodologies were more preferable for observing temporal trends in animal biomass within a country. The FDA’s and OIE’s parameters can track year-specific weight differentiations over time as the denominators of both methodologies estimate animal weights annually; however, the ESVAC’s and PHAC’s denominators cannot track year-specific weight differentiations due to the use of standardized weight parameters (Figure 8; worst in sub-criterion Sw, Table S21). Therefore, unlike the FDA’s and OIE’s weight parameters, the ESVAC’s and PHAC’s standard weights should be updated as time passes. Overall, all denominators are similar in their stability in population (So), but the difference in weight parameters favours the FDA and OIE methodologies.
Characteristics of an ideal biomass methodology depend on its intended use; however, there are some characteristics we think are necessary to estimate the biomass-adjusted sales as identified through application of our evaluation sub-criteria on the biomass denominators (Table 1).38,85 Due to limitations observed in most sub-criteria, the animal biomass estimates from the FDA, ESVAC, PHAC and OIE methodologies may lead to an under- or over-estimation of the biomass-adjusted antimicrobial sales in animals, which would reduce the usefulness of the metrics for any objective. In that regard, two important limitations of the biomass denominator, related to the data availability, were identified in this study. The first limitation concerns data availability for the estimations of biomass denominators (sub-criteria G and C): population and weight data used for biomass denominators are estimated from available databases, and are dependent on the ability of the surveys to represent the actual animal population and weight.34–37 An implication of this limitation was the underestimation of the annual cattle population in the USA, which resulted from the lack of compatibility between the databases. Specifically, cattle biomass estimation for all methodologies (FDA, ESVAC, PHAC and OIE) requires the use of Livestock Slaughter Summaries as well as Cattle Inventory databases to estimate the biomass of slaughtered and livingstock animals; however, the animals counted in these two databases overlap, and a clear estimate of the population size cannot be obtained from the reports. For now, our solution to this problem about cattle population was to include animal categories only when it was certain that the categories are counted only once. This limitation resulted in an underestimation of the cattle biomass and consequently an overestimation of the biomass-adjusted antimicrobial sales. Using the FDA methodology and assuming an unlikely scenario of zero overlap between databases, at most, the biomass-adjusted sales in 2018 could have been about 10% over-represented, considering that we included 73.2 million cattle in the biomass estimation, in contrast to the 94.8 million living cattle reported by the Cattle Inventory.50 However, even if that were the case, it would not change the ranking of the different biomass-adjusted sales estimates, and, therefore, can be considered negligible from this point of view. The second limitation is related to the weight estimation and the use of average weights at slaughter and at treatment (sub-criterion A), which was discussed earlier and does not accurately account for the weight of animals at the time of antimicrobial administration since different diseases affect species at different ages and weights. Considering the two limitations in the biomass denominators mentioned above, caution is warranted in interpreting and comparing adjusted and unadjusted species-specific sales estimates.33
Additional major limitations of the four evaluated methodologies stem from their use of the antimicrobial sales in the numerator. Importantly, antimicrobial sales data are prone to reporting bias because these data are collected from antimicrobial drug manufacturers, who are required to report to the FDA the amount of antimicrobials they sell or distribute for use in food-producing animals.86 Another limitation concerns an implicit assumption that is necessary when comparing antimicrobial sales (adjusted or unadjusted for biomass) among countries. While this limitation did not affect the results for biomass-adjusted sales for the USA in this study, comparing antimicrobial sales across countries ignores any lack of synchronization of approvals on antimicrobials and other discrepancies among countries.36,37 Importantly, sales data provide no information about the actual animals’ exposure to antimicrobials, such as in terms of diseases indication, age/weight of treatment, dose frequency or course duration.87,88 The actual antimicrobial exposure for a disease depends on potency/molecular weight and doses, which vary by antimicrobial,78,89 animal species, production categories39 and administration route,90 but the sales data do not reflect those differences. Thus, while the biomass-adjusted antimicrobial sales estimated by the four methodologies are valuable, they do not reflect the actual use of antimicrobials in animals, and, as such, antimicrobial stewardship cannot be inferred from the biomass-adjusted sales estimates.
Several existing studies have focused on improving monitoring of antimicrobial use and antimicrobial sales to address the limitations of the current monitoring strategies. Regarding monitoring of the actual antimicrobial use, in the USA, Schrag et al.87 (2020) proposed a method for quantifying antimicrobial use in dairy cattle by accounting for the dose of an antimicrobial, course duration and dose frequency. In Canada (by PHAC) and Germany, metrics were developed which use the DDD for tracking antimicrobial use at the herd level.24,36,41 Mills et al.38 (2018) accounted for daily doses as well as the course length of antimicrobials in dairy cattle in the UK. The studies including dose-related information are scarce, as this kind of information is not always available at the country-, species- and antimicrobial-level, since data collection is costly and time-consuming.91 Nevertheless, countries like the Netherlands and Denmark have been able to introduce the DDD concept in national benchmarking systems.92–94 On the other hand, to improve monitoring of antimicrobial sales, the methodology developed by Radke95 (2017) accounts for the growth cycle or lifespan of animal species and re-standardizes the animal weights used in the PCUEU calculation. With Radke’s methodology, animal weights at risk are better represented due to inclusion of weight data at different stages of animal life; however, accounting for a single growth cycle is not compatible with the standard antimicrobial sales data that usually represent annual sales, thus using that approach would further complicate the interpretation of antimicrobial sales.
In conclusion, the current study estimated the biomass-adjusted antimicrobial sales for use in the major food-producing animals in the USA using the biomass methodologies established by the FDA, ESVAC, PHAC and OIE. The four methodologies resulted in substantially different estimates of biomass-adjusted antimicrobial sales. While the biomass denominator of each methodology has some advantages over the other denominators, at present none meets all the needs of a metric for estimating animal biomass and consequently for monitoring veterinary antimicrobial sales. The review and comparison of the biomass denominators of the four methodologies identified their strengths and weaknesses and revealed the causes for differences in their biomass-adjusted estimates of antimicrobial sales. This will allow for a better interpretation and comparison of antimicrobial sales data across different methodologies. The main source of the observed differences was the different animal weight parameters used in the biomass calculation. Thus, global efforts to streamline the methodologies to monitor antimicrobial sales in food-producing animals could prioritize weight parameters for standardization. This work presents an essential step in that direction.
Supplementary Material
Acknowledgements
We thank representative experts Susan J. Bright-Ponte (Veterinary Medical Officer, FDA Center for Veterinary Medicine), Kristine Ignate (Scientific Administrator, European Medicines Agency Surveillance and Regulatory Support Department), Carolee Carson (Veterinary Epidemiologist, Public Health Agency of Canada) and Agnes Agunos (Veterinary Epidemiologist, Public Health Agency of Canada), and Morgan Jeannin (Antimicrobial Use Team, OIE Antimicrobial Resistance and Veterinary Products Department) who provided insights into the FDA, ESVAC, PHAC and OIE methodologies, respectively. We also thank Travis Averill (Livestock Branch Chief, USDA NASS Statistics Division) and Grace Grossen (Agricultural Economist, USDA ERS Market and Trade Economics Division) for their help in navigating the USDA databases. Finally, we would like to thank Hannah Padda and Michelle Wemette for helpful comments on the manuscript.
Funding
Support for this project was provided by the Pew Charitable Trusts. The views expressed herein are those of the authors and do not necessarily reflect the views of the Pew Charitable Trusts.
Transparency declarations
None to declare.
Author contributions
R.I. and E.B. designed the research; E.B. performed the research, analysed the data and drafted the manuscript with assistance from R.I.; E.B. and R.I. finalized and approved the manuscript.
Supplementary data
Tables S1 to S21 and Figures S1 to S9 are available as Supplementary data at JAC Online.
References
- 1. WHO. Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- 2. Hoelzer K, Wong N, Thomas J. et al. Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res 2017; 13: 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marshall BM, Levy SB.. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 2011; 24: 718–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conrad CC, Stanford K, Narvaez-Bravo C. et al. Farm fairs and petting zoos: a review of animal contact as a source of zoonotic enteric disease. Foodborne Pathog Dis 2017; 14: 59–73. [DOI] [PubMed] [Google Scholar]
- 5. Karon AE, Archer JR, Sotir MJ. et al. Human multidrug-resistant Salmonella Newport infections, Wisconsin, 2003-2005. Emerg Infect Dis 2007; 13: 1777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramchandani M, Manges AR, DebRoy C. et al. Possible animal origin of human-associated, multidrug-resistant, uropathogenic Escherichia coli. Clin Infect Dis 2005; 40: 251–7. [DOI] [PubMed] [Google Scholar]
- 7. Varma JK, Marcus R, Stenzel SA. et al. Highly resistant Salmonella Newport-MDRAmpC transmitted through the domestic US food supply: a FoodNet case-control study of sporadic Salmonella Newport infections, 2002–2003. J Infect Dis 2006; 194: 222–30. [DOI] [PubMed] [Google Scholar]
- 8. Zhao S, Qaiyumi S, Friedman S. et al. Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J Clin Microbiol 2003; 41: 5366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winokur PL, Vonstein DL, Hoffman LJ. et al. Evidence for transfer of CMY-2 ampc β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob Agents Chemother 2001; 45: 2716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graham JP, Evans SL, Price LB. et al. Fate of antimicrobial-resistant enterococci and staphylococci and resistance determinants in stored poultry litter. Environ Res 2009; 109: 682–9. [DOI] [PubMed] [Google Scholar]
- 11. O’Neill J. The review on antimicrobial resistance - antimicrobials in agriculture and the environment: reducing unnecessary use and waste. https://ec.europa.eu/health/sites/health/files/antimicrobial_resistance/docs/amr_studies_2015_am-in-agri-and-env.pdf.
- 12. Aarestrup FM, Seyfarth AM, Emborg HD. et al. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother 2001; 45: 2054–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klare I, Badstübner D, Konstabel C. et al. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb Drug Resist 1999; 5: 45–52. [DOI] [PubMed] [Google Scholar]
- 14. Endtz HP, Ruijs GJ, van Klingeren B. et al. Quinolone resistance in Campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Antimicrob Chemother 1991; 27: 199–208. [DOI] [PubMed] [Google Scholar]
- 15. Sánchez R, Fernández-Baca V, Díaz MD. et al. Evolution of susceptibilities of Campylobacter spp. to quinolones and macrolides. Antimicrob Agents Chemother 1994; 38: 1879–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith KE, Besser JM, Hedberg CW. et al. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N Engl J Med 1999; 340: 1525–32. [DOI] [PubMed] [Google Scholar]
- 17. Van Boeckel TP, Glennon EE, Chen D. et al. Reducing antimicrobial use in food animals. Science 2017; 357: 1350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin MJ, Thottathil SE, Newman TB.. Antibiotics overuse in animal agriculture: a call to action for health care providers. Am J Public Health 2015; 105: 2409–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Boeckel TP, Brower C, Gilbert M. et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 2015; 112: 5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. European Commission. Ban on antibiotics as growth promoters in animal feed enters into effect. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjvvqaR3Y_wAhWLQc0KHeLGAYIQFjAAegQIBhAD&url=https%3A%2F%2Fec.europa.eu%2Fcommission%2Fpresscorner%2Fapi%2Ffiles%2Fdocument%2Fprint%2Fen%2Fip_05_1687%2FIP_05_1687_EN.pdf&usg=AOvVaw0bgX2vf5RRzkofMBROUqU-.
- 21. FDA. Fact sheet: Veterinary feed directive final rule and next steps. https://www.fda.gov/animal-veterinary/development-approval-process/fact-sheet-veterinary-feed-directive-final-rule-and-next-steps.
- 22. Government of Canada. Antimicrobial resistance and animals - actions. https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/actions.html#a1.
- 23. WHO. Global action plan on antimicrobial resistance. https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf;jsessionid=6CA4CA16DA52A80A5AEC2121760241F2?sequence=1.
- 24. Lekagul A, Tangcharoensathien V, Yeung S.. The use of antimicrobials in global pig production: a systematic review of methods for quantification. Prev Vet Med 2018; 160: 85–98. [DOI] [PubMed] [Google Scholar]
- 25. The Pew Charitable Trusts. FDA proposal would support fight against antibiotic-resistant bacteria. https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2019/06/fda-proposal-would-support-fight-against-antibiotic-resistant-bacteria.
- 26. Collineau L, Belloc C, Stärk KDC. et al. Guidance on the selection of appropriate indicators for quantification of antimicrobial usage in humans and animals. Zoonoses Public Health 2017; 64: 165–84. [DOI] [PubMed] [Google Scholar]
- 27. WHO. Integrated surveillance of antimicrobial resistance: Guidance from a WHO advisory group (AGISAR). Geneva: WHO. 2013. https://apps.who.int/iris/handle/10665/91778.
- 28. Merle R, Meyer-Kühling B.. Sales data as a measure of antibiotics usage: concepts, examples and discussion of influencing factors. Vet Med Sci 2020; 6: 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tiseo K, Huber L, Gilbert M. et al. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics (Basel) 2020; 9: 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Köper LM, Bode C, Bender A. et al. Eight years of sales surveillance of antimicrobials for veterinary use in Germany—what are the perceptions? PLoS One 2020; 15: e0237459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Werner N, McEwen S, Kreienbrock L. et al. Monitoring antimicrobial drug usage in animals: methods and applications. Microbiol Spectr 2018; 10.1128/microbiolspec.ARBA-0015-2017. [DOI] [PubMed] [Google Scholar]
- 32. Bright-Ponte SJ. Antimicrobial use data collection in animal agriculture. Zoonoses Public Health 2020; 67: 1–5. [DOI] [PubMed] [Google Scholar]
- 33. FDA. FDA releases annual summary report on antimicrobials sold or distributed in 2019 for use in food-producing animals. https://www.fda.gov/animal-veterinary/cvm-updates/fda-releases-annual-summary-report-antimicrobials-sold-or-distributed-2019-use-food-producing.
- 34. EMA. European surveillance of veterinary antimicrobial consumption - sales of veterinary antimicrobial agents in 31 European countries in 2017 (EMA/294674/2019). https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2017_en.pdf.
- 35. FDA. FDA’s proposed method for adjusting data on antimicrobials sold or distributed for use in food-producing animals, using a biomass denominator. https://www.fda.gov/files/animal%20&%20veterinary/published/FDA%E2%80%99s-Proposed-Method-for-Adjusting-Data-on-Antimicrobials-Sold-or-Distributed-for-Use-in-Food-Producing-Animals-Using-a-Biomass-Denominator–Technical-Paper.pdf.
- 36. Government of Canada. Canadian integrated program for antimicrobial resistance surveillance (CIPARS) 2016 annual report. Public Health Agency of Canada, Guelph, Ontario.http://publications.gc.ca/collections/collection_2018/aspc-phac/HP2-4-2016-eng.pdf.
- 37. World Organisation for Animal Health. OIE annual report on antimicrobial agents intended for use in animals - fourth report. https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/A_Fourth_Annual_Report_AMU.pdf. [DOI] [PMC free article] [PubMed]
- 38. Mills HL, Turner A, Morgans L. et al. Evaluation of metrics for benchmarking antimicrobial use in the UK dairy industry. Vet Rec 2018; 182: 379. [DOI] [PubMed] [Google Scholar]
- 39. Bondt N, Jensen VF, Puister-Jansen LF. et al. Comparing antimicrobial exposure based on sales data. Prev Vet Med 2013; 108: 10–20. [DOI] [PubMed] [Google Scholar]
- 40. Brault SA, Hannon SJ, Gow SP. et al. Calculation of antimicrobial use indicators in beef feedlots—effects of choice of metric and standardized values. Front Vet Sci 2019; 6: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kasabova S, Hartmann M, Werner N. et al. Used daily dose vs. defined daily dose—contrasting two different methods to measure antibiotic consumption at the farm level. Front Vet Sci 2019; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grave K, Torren-Edo J, Muller A. et al. Variations in the sales and sales patterns of veterinary antimicrobial agents in 25 European countries. J Antimicrob Chemother 2014; 69: 2284–91. [DOI] [PubMed] [Google Scholar]
- 43. Agunos A, Léger DF, Carson CA. et al. Antimicrobial use surveillance in broiler chicken flocks in Canada, 2013-2015. PLoS One 2017; 12: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Agunos A, Gow SP, Léger DF. et al. Antimicrobial use and antimicrobial resistance indicators-integration of farm-level surveillance data from broiler chickens and turkeys in British Columbia, Canada . Front Vet Sci 2019; 6: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Góchez D, Raicek M, Pinto Ferreira J. et al. OIE annual report on antimicrobial agents intended for use in animals: methods used. Front Vet Sci 2019; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. FDA. 2018 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. https://www.fda.gov/media/133411/download.
- 47. WHO. Critically important antimicrobials for human medicine, 6th revision. https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf.
- 48. USDA NASS. Livestock slaughter 2018 summary, released 04/2019. https://www.nass.usda.gov/Publications/Todays_Reports/reports/lsslan19.pdf.
- 49. USDA NASS. Livestock slaughter 2017 summary, released 04/2018. https://downloads.usda.library.cornell.edu/usda-esmis/files/r207tp32d/cn69m6457/pc289m639/LiveSlauSu-04-18-2018.pdf.
- 50. USDA NASS. Cattle inventory, released 02/28/2019. https://www.nass.usda.gov/Publications/Todays_Reports/reports/catl0219.pdf.
- 51. USDA NASS. Cattle inventory, released 01/31/2018. https://www.nass.usda.gov/Publications/Todays_Reports/reports/catl0120.pdf.
- 52. USDA NASS. Quarterly hogs and pigs inventory, released 06/27/2019. https://www.nass.usda.gov/Publications/Todays_Reports/reports/hgpg0619.pdf.
- 53. USDA NASS. Quarterly hogs and pigs inventory, released 09/27/2018. https://downloads.usda.library.cornell.edu/usda-esmis/files/rj430453j/ht24wz08d/1v53k967v/hgpg0919.pdf.
- 54. USDA NASS. Quarterly hogs and pigs inventory, released 12/22/2017. https://www.nass.usda.gov/Publications/Todays_Reports/reports/hgpg1217.pdf.
- 55. USDA NASS. Poultry slaughter 2018 summary, released 04/2019. https://www.nass.usda.gov/Publications/Todays_Reports/reports/pslaan19.pdf.
- 56. USDA NASS. Poultry slaughter 2017 summary, released 12/2018. https://downloads.usda.library.cornell.edu/usda-esmis/files/pg15bd88s/br86b638d/4f16c564f/PoulSlauSu-02-26-2018.pdf.
- 57. USDA ERS. Livestock and meat international trade data - annual and cumulative year-to-date US livestock and meat trade by country, updated on 08/06/2020. https://www.ers.usda.gov/data-products/livestock-and-meat-international-trade-data/.
- 58. USDA FAS. Global agricultural trade system. https://apps.fas.usda.gov/gats/default.aspx.
- 59. USDA APHIS. USDA APHIS NAHMS beef 2007-08 part iv: Reference of beef cow-calf management practices in the United States, 2007–08https://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef0708/Beef0708_dr_PartIV_1.pdf.
- 60. Bovine Alliance on Management and Nutrition. A BAMN publication - heifer growth and economics: Target growth. https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/bamn/BAMN07_HeiferGrowth.pdf.
- 61. Lawrence JD, Ellis S, Iowa State University Extension and Outreach, ag decision maker - returns from farrowing and finishing hogs. https://www.extension.iastate.edu/agdm/livestock/pdf/b1-30.pdf.
- 62. World Animal Health Information Database. United States of America, animal population summary: All species, 2018. https://www.oie.int/wahis_2/public/wahidphp/Countryinformation/Animalpopulation.
- 63. FAOSTAT. Livestock primary, producing animals/slaughtered. http://www.fao.org/faostat/en/#data/QL.
- 64. EUROSTAT. Manual for the compilation of supply balance sheets for meat. https://circabc.europa.eu/sd/a/90447c6f-5b7c-4b6f-87e9-27c5a7a5c923/ASA-TE-F-655%2520SBS%2520Manual%2520-%2520meat.doc.
- 65. FDA. CVM GFI #209 the judicious use of medically important antimicrobial drugs in food-producing animals. 2012. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-209-judicious-use-medically-important-antimicrobial-drugs-food-producing-animals.
- 66. FDA. CVM GFI #213 new animal drugs and new animal drug combination products administered in or on medicated feed or drinking water of food-producing animals: Recommendations for drug sponsors for voluntarily aligning product use conditions with GFI #209. 2013. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-213-new-animal-drugs-and-new-animal-drug-combination-products-administered-or-medicated-feed.
- 67. Grave K, Jensen VF, Odensvik K. et al. Usage of veterinary therapeutic antimicrobials in Denmark, Norway and Sweden following termination of antimicrobial growth promoter use. Prev Vet Med 2006; 75: 123–32. [DOI] [PubMed] [Google Scholar]
- 68. Grave K, Kaldhusdal MC, Kruse H. et al. What has happened in Norway after the ban of avoparcin? Consumption of antimicrobials by poultry. Prev Vet Med 2004; 62: 59–72. [DOI] [PubMed] [Google Scholar]
- 69. McEwen SA, Angulo FJ, Collignon PJ. et al. Unintended consequences associated with national-level restrictions on antimicrobial use in food-producing animals. Lancet Planet Health 2018; 2: e279–e82. [DOI] [PubMed] [Google Scholar]
- 70. WHO. The WHO international review panel’s evaluation of the termination of the use of antimicrobial growth promoters in Denmark: Foulum, Denmark, 6–9 November 2002. https://apps.who.int/iris/handle/10665/68357.
- 71. Mevius D, Koene M, Wit B. et al. Maran 2008: Monitoring of antimicrobial resistance and antibiotic usage in animals in the Netherlands in 2008. https://www.wur.nl/upload_mm/7/6/a/5becb8c3-70be-4930-ae2f-397760c2b440_MARAN2008.pdf.
- 72. de Jong I, Bondt N, Ge L. et al. Reduction in antibiotic usage in broilers: Side effects and best practices. http://edepot.wur.nl/253812.
- 73. Wierup M. The Swedish experience of the 1986 year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb Drug Resist 2001; 7: 183–90. [DOI] [PubMed] [Google Scholar]
- 74. Aarestrup FM, Jensen VF, Emborg H-D. et al. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am J Vet Res 2010; 71: 726–33. [DOI] [PubMed] [Google Scholar]
- 75. Ahlmeyer V, Clark S. Proceedings, annual meeting of the United States animal health association - turkey industry report. https://www.poultrymed.com/Poultrymed/userdata/SendFile.asp?DBID=1&LNGID=1&GID=2653.
- 76. Clark SR, Bailey A. Proceedings 118th annual meeting of the USAHA, Kansas City, MO; transmissible diseases of poultry and other avian species committee - turkey industry annual report - current health and industry issues facing the US turkey industry. https://www.usaha.org/upload/Proceedings/2018_Proceedings_v2_Comb_FINAL.pdf.
- 77. USPOULTRY. Antimicrobial use in poultry - antimicrobial stewardship within US poultry production, 2013–2017 report. https://www.uspoultry.org/poultry-antimicrobial-use-report/docs/USPOULTRY_Antimicrobial-Report.pdf.
- 78. Singer RS, Porter LJ, Schrag NFD. et al. Estimates of on‐farm antimicrobial usage in turkey production in the United States, 2013–2017. Zoonoses Public Health 2020; 67: 36–50. [DOI] [PubMed] [Google Scholar]
- 79. Froebel L, Clark S, Turkey industry report - report of the USAHA committee on poultry and other avian species. https://www.usaha.org/upload/Committee/2019Reports/Poultry_and_Other_Avian_Species_.pdf.
- 80. Kim YS, Fukumoto GK, Kim S.. Carcass quality and meat tenderness of Hawaii pasture-finished cattle and Hawaii-originated, mainland feedlot-finished cattle. Trop Anim Health Prod 2012; 44: 1411–5. [DOI] [PubMed] [Google Scholar]
- 81. Mwangi FW, Charmley E, Gardiner CP. et al. Diet and genetics influence beef cattle performance and meat quality characteristics. Foods 2019; 8: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. FAO. Mitigating the impacts of Covid-19 on the livestock sector http://www.fao.org/3/ca8799en/CA8799EN.pdf.
- 83. WHO. WHO report on surveillance of antibiotic consumption: 2016-2018 early implementation. Geneva: WHO. Licence: Cc BY-NC-SA 3.0 igo. 2018. https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf.
- 84. WHO. Antimicrobial resistance—global report on surveillance. https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf?sequence=1.
- 85. Benedict KM, Gow SP, Reid-Smith RJ. et al. Metrics for quantifying antimicrobial use in beef feedlots. Can Vet J 2012; 53: 841–8. [PMC free article] [PubMed] [Google Scholar]
- 86. FDA. Questions and answers: Summary report on antimicrobials sold or distributed for use in food-producing animals. 2020. https://www.fda.gov/industry/animal-drug-user-fee-act-adufa/questions-and-answers-summary-report-antimicrobials-sold-or-distributed-use-food-producing-animals.
- 87. Schrag NFD, Apley MD, Godden SM. et al. Antimicrobial use quantification in adult dairy cows – part 1 – standardized regimens as a method for describing antimicrobial use. Zoonoses Public Health 2020; 67: 51–68. [DOI] [PubMed] [Google Scholar]
- 88. Schrag NFD, Apley MD, Godden SM. et al. Antimicrobial use quantification in adult dairy cows – part 2 – developing a foundation for pharmacoepidemiology by comparing measurement methods. Zoonoses Public Health 2020; 67: 69–81. [DOI] [PubMed] [Google Scholar]
- 89. Singer RS, Porter L, Schrag NFD. et al. Estimates of on-farm antimicrobial usage in broiler chicken production in the United States, 2013 – 2017. Zoonoses Public Health 2020; 67: 22–35. [DOI] [PubMed] [Google Scholar]
- 90. EMA. Defined Daily Doses for animals (DDDvet) and Defined Course Doses for animals (DCDvet) - European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). London (EMA/224954/2016). https://www.ema.europa.eu/en/documents/other/defined-daily-doses-animals-dddvet-defined-course-doses-animals-dcdvet-european-surveillance_en.pdf.
- 91. Schar D, Sommanustweechai A, Laxminarayan R. et al. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: optimizing use and addressing antimicrobial resistance. PLoS Med 2018; 15: e1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bos MEH, Mevius DJ, Wagenaar JA. et al. Antimicrobial prescription patterns of veterinarians: introduction of a benchmarking approach. J Antimicrob Chemother 2015; 70: 2423–5. [DOI] [PubMed] [Google Scholar]
- 93. DANMAP. DANMAP 2015 - use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark - microbiology and infection control, Statens Serum Institute; National Food Institute; Technical University of Denmark. https://www.food.dtu.dk/english/-/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2016/Report-DANMAP-2015.ashx?la=da&hash=5DBBDBE2C00428F554EB2A215F6940AFE02FA6F4.
- 94. More SJ. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir Vet J 2020; 73: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Radke BR. Towards an improved estimate of antimicrobial use in animals: adjusting the "population correction unit" calculation. Can J Vet Res 2017; 81: 235–40. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.