Abstract
Background:
Approximately 80% of the 463 million adults worldwide with diabetes live in low- and middle-income countries (LMICs). A major obstacle to designing evidence-based policies to improve diabetes outcomes in LMICs is the limited nationally representative data on the current patterns of treatment coverage. The objectives of this study are (1) to estimate the proportion of adults with diabetes in LMICs who receive coverage of recommended pharmacological and non-pharmacological diabetes treatment and (2) to describe country-level and individual-level characteristics that are associated with treatment.
Methods:
We conducted a cross-sectional analysis of pooled, individual data from 55 nationally representative surveys in LMICs. Our primary outcome of self-reported diabetes treatment coverage was based upon population-level monitoring indicators recommended in the 2020 World Health Organization Package of Essential Noncommunicable Disease Interventions. We assessed coverage of three pharmacological and three non-pharmacological treatments among people with diabetes. At the country level, we estimated the proportion of individuals reporting coverage by per-capita gross national income and geographic region. At the individual level, we used logistic regression models to assess coverage along several key individual characteristics including sex, age, BMI, wealth quintile, and educational attainment. In the primary analysis, we scaled sample weights such that countries were weighted equally.
Findings:
The final pooled sample from the 55 LMICs included 680,102 total individuals and 37,094 individuals with diabetes. Using equal weights for each country, diabetes prevalence was 9.0% (95% confidence interval [CI], 8.7–9.4), with 43.9% (95% CI, 41.9–45.9) reporting a prior diabetes diagnosis. Overall, 4.6% (95% CI, 3.9–5.4) of individuals with diabetes self-reported meeting need for all treatments recommended for them. Coverage of glucose-lowering medication was 50.5% (95% CI, 48.6–52.5); antihypertensive medication, 41.3% (95% CI, 39.3–43.3); cholesterol-lowering medication, 6.3% (95% CI, 5.5–7.2); diet counseling, 32.2% (95% CI, 30.7–33.7); exercise counseling, 28.2% (95% CI, 26.6–29.8); and weight-loss counseling, 31.5% (95% CI, 29.3–33.7). Countries at higher income levels tended to have greater coverage. Female sex and higher age, BMI, educational attainment, and household wealth were also associated with greater coverage.
Interpretation:
Fewer than one in ten people with diabetes in LMICs receive coverage of guideline-based comprehensive diabetes treatment. Scaling-up the capacity of health systems to deliver treatment not only to lower glucose but also to address cardiovascular disease risk factors such as hypertension and high cholesterol are urgent global diabetes priorities.
INTRODUCTION
The global burden of disability-adjusted life years attributable to diabetes and elevated fasting glucose has more than doubled from 1990–2019.1 Approximately 80% of the estimated 463 million adults with diabetes worldwide live in low- and middle-income countries (LMICs).2 The limitations of health systems in LMICs to deliver treatment for non-communicable diseases (NCDs) like diabetes leads to substantial excess mortality.3,4 Expanding access to diabetes treatment is critical for many countries to meet Sustainable Development Goal 3.4 (reduce premature mortality from NCDs by one-third by 2030).5
The World Health Organization (WHO) recommends a comprehensive approach to clinical care of type 2 diabetes consisting of pharmacological and non-pharmacological treatment targeting glycemic control and key cardiovascular disease (CVD) risk factors such as hypertension, elevated cholesterol, and obesity.6,7 This approach is supported by randomized clinical trials and high-quality cohort studies showing the benefit of multiple risk factor reduction among people with diabetes.3,8,9 Within this paradigm, the pharmacological treatments shown to be most effective in improving diabetes outcomes include glucose-lowering medications, antihypertensive medications, and cholesterol-lowering medications.10 Non-pharmacological treatments such as counseling on diet, exercise, and weight loss are widely recommended in guidelines, though their role is less well defined given a paucity of data supporting long-term effectiveness, especially in LMICs.10–12
Overall, there are limited nationally representative data on the performance of health systems in LMICs in delivering comprehensive diabetes treatment.13 A prior study assessing diabetes care cascades using individual data from 28 nationally representative surveys in LMICs demonstrated substantial gaps in glycemic care, but this study did not explore other components of diabetes treatment such as use of antihypertensive or cholesterol-lowering medication.14 National and regional strategies to treat the growing number of people with diabetes in LMICs should be shaped by the best available evidence on the coverage and quality of diabetes treatment.15
The objectives of this study are (1) to estimate the proportion of adults with diabetes in LMICs who receive coverage of recommended pharmacological and non-pharmacological diabetes treatment and (2) to describe country-level and individual-level characteristics that are associated with treatment. Our estimates can be used by policymakers in LMICs to prioritize resources, target interventions, and benchmark progress in scaling-up comprehensive diabetes treatment.
METHODS
Study design
We conducted a cross-sectional analysis of pooled, individual-level data from 55 nationally representative surveys in LMICs. Surveys were included if they were conducted in 2008 or after in an LMIC as classified by the World Bank in the year the survey was conducted, were nationally representative, had individual-level data, contained a diabetes biomarker (fasting glucose, random glucose, or HbA1c), and had data on one or more diabetes treatments as defined below.
Our two-step process for pooling surveys has been described previously.16 First, we identified all LMICs in which a WHO Stepwise Approach to Surveillance (STEPS) survey had been conducted. Prior to 2019, we requested each STEPS survey from a list maintained on the WHO website. Beginning in 2019, we downloaded STEPS surveys from the WHO Central Data Catalog. Second, for countries in which no eligible STEPS survey was available or accessible, we conducted a systematic Google search in April 2020 to identify non-STEPS surveys. In total, we included STEPS surveys from 44 countries and non-STEPS surveys from 11 countries. See appendix p 3-35 for further information on our search process, a map of included countries, and details of included surveys.
Sample and definitions
Our sample included non-pregnant individuals with an available diabetes biomarker who were at least 25 years of age. We excluded people younger than 25 years of age as this was the minimum age for inclusion in many surveys.
Diabetes status was defined by self-reported use of a glucose-lowering medication (oral glucose-lowering medication or insulin) or biochemical evidence of diabetes using the WHO definition: fasting plasma glucose (FPG) ≥7.0 mmol/l (126 mg/dl), random plasma glucose ≥11.1 mmol/l (200 mg/dl), or an HbA1c measurement ≥6.5%.6 The diabetes biomarker used for diagnosis was a point-of-care fasting capillary glucose in 44 surveys, a laboratory-based fasting plasma glucose in seven surveys, and HbA1c in four surveys. In countries with capillary glucose measurements, we converted values to plasma glucose by multiplying by a factor of 1.11 based on research showing that capillary glucose underestimates plasma levels.17 Where fasting status was missing, with one exception, we assumed that the glucose measurement was fasting in accordance with survey protocols. The exception was India where random blood glucose was the primary diabetes biomarker.
Hypertensive status was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or self-reported use of an antihypertensive medication.6 For respondents with three blood pressure measurements, values were averaged over the final two readings. For respondents with two blood pressure measurements, we used the mean of the two values. See appendix p 36-40 for additional information on diabetes biomarker and blood pressure measurements.
Outcomes
Our primary outcome of diabetes treatment coverage was based on population-level monitoring indicators recommended in the 2020 WHO Package of Essential Noncommunicable Disease Interventions (WHO PEN).6 Consistent with WHO PEN, we defined diabetes treatment coverage as the “proportion of eligible persons receiving drug therapy and counseling (including glycemic control) to prevent heart attacks and strokes” in population-based data sources such as STEPS surveys.6 We estimated self-reported coverage for six core type 2 diabetes clinical treatments recommended in WHO PEN.6 The first group consisted of three pharmacological treatments: (1) glucose-lowering, (2) antihypertensive, and (3) cholesterol-lowering medication. The second group consisted of three non-pharmacological treatments: counseling on (4) diet, (5) exercise, and (6) weight loss.
We used WHO PEN to define the numerator and denominator for each outcome (Table 1 and appendix p 41-44).6 Glucose-lowering medication use was quantified among individuals not achieving glycemic targets or self-reporting use of an oral glucose-lowering medication or insulin. We set glycemic targets of HbA1c <7.0% (equivalent to FPG <8.0 mmol/l18) in people younger than 65 years of age and HbA1c <8.0% (FPG <9.2 mmol/l) in people 65 years or older. Antihypertensive medication use was quantified among people with both diabetes and hypertension. Cholesterol-lowering medication use was quantified among individuals with diabetes over 40 years of age; we did not assess serum cholesterol levels as lipid measurements are not required to guide cholesterol-lowering therapy according to the WHO PEN guidelines. Counseling on diet and exercise was quantified among all people with diabetes. Counseling on weight-loss was quantified among individuals with diabetes and body mass index (BMI) ≥25 kg/m2.
Table 1: Definitions of outcomes.
Outcome definitions are consistent with recommended population-level monitoring indicators and clinical treatment guidelines in the 2020 World Health Package of Essential Noncommunicable Disease Interventions.6 BMI=body mass index.
| Indicator | Glucose-lowering medication | Antihypertensive medication | Cholesterol-lowering medication | Diet counseling | Exercise counseling | Weight-loss counseling |
|---|---|---|---|---|---|---|
| Countries, n | 55 | 52 | 46 | 49 | 48 | 48 |
| Numerator | Individuals self-reporting use of an oral glucose-lowering medication or insulin | Individuals self-reporting use of an antihypertensive medication | Individuals self-reporting use of a cholesterol-lowering medication. | Individuals self-reporting dietary counseling | Individuals self-reporting exercise counseling | Individuals self-reporting weight-loss counseling |
| Denominator | Individuals with diabetes not achieving glycemic target or self-reporting use of a glucose-lowering medication | Individuals with diabetes and hypertension | Individuals with diabetes age 40 years or older | Individuals with diabetes | Individuals with diabetes | Individuals with diabetes and BMI ≥25 kg/m2 |
Statistical analyses
We estimated the proportion of individuals self-reporting coverage of each diabetes treatment alone and in combination. We also estimated coverage by country per-capita gross national income (GNI) and geographic region adapted from the classification scheme of the NCD Risk Factor Collaboration.19 A country’s GNI was included both as a continuous variable and in categories defined by the World Bank in the year the survey was conducted.
We then assessed coverage along several key individual-level characteristics. Specifically, we constructed univariable and multivariable logistic regression models with country-level fixed effects and individual-level covariates of sex, age, BMI, wealth quintile, and educational attainment. Age and BMI were included as continuous variables using restricted cubic splines with five knots at 5%, 27.5%, 50%, 72.5%, and 95%. Household wealth quintiles were based on asset indices or income depending on the available data in each survey (appendix p 45). Survey weighting and clustering at the country level were accounted for in all analyses. We used the sample weights for the diabetes biomarker when available. Given that our main interest was at the level of the health system, we scaled sample weights such that countries were weighted equally. The India survey therefore contributes equally to other countries despite its large sample size. We used a complete case analysis in the models. Overall, 7.9% of participants were missing data on at least one individual-level characteristic (appendix p 46-51). We report model outputs as predicted probabilities. Analyses were performed in Stata version 16.1.
Sensitivity analyses
We conducted multiple sensitivity analyses. First, we restricted the sample to individuals reporting a prior diabetes diagnosis. Second, we weighted each country in proportion to its 2015 population size. Third, given differences in the upper age limit of surveys, we limited the analysis to individuals ages 25 to 64 years. Fourth, the WHO recommends using CVD risk scores to guide treatment with cholesterol-lowering medications when it is infeasible to treat all people with diabetes over 40 years of age.7 Therefore, we estimated coverage of cholesterol-lowering medication among people with diabetes who have a 10-year predicted CVD risk ≥20% using the 2019 WHO non-laboratory risk equations.20 Finally, we re-specified the multivariable logistic regressions as multilevel models using random effects at the country level.
Ethics
Our use of de-identified survey data was determined not to be human subjects research by the institutional review board of the University of Michigan.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or submitting of the report for publication. All authors had full access to the data and approved the final version.
RESULTS
The final pooled sample from the 55 LMICs included 680,102 total individuals and 37,094 individuals with diabetes (Table 2; detailed sample characteristics are reported in appendix p 52). Using equal weights for each country, diabetes prevalence by self-report of a glucose-lowering medication or biomarker was 9.0% (95% confidence interval [CI], 8.7–9.4), with 43.9% (95% CI, 41.9–45.9) reporting a prior diabetes diagnosis. Diabetes prevalence and the proportion of diagnosed diabetes tended to be greater in countries in higher World Bank income categories (appendix p 53-55). Among geographic regions, Oceania had the highest diabetes prevalence (19.3% [95% CI, 18.0–20.7]) and lowest proportion diagnosed (29.7% [95% CI, 23.7–36.6]). Latin America and the Caribbean had the next highest diabetes prevalence (17.4% [95% CI, 16.1–18.8]) but had the highest proportion diagnosed (66.8% [95% CI, 62.9–70.5]).
Table 2: Survey characteristics.
Country income group as classified by the World Bank in the year the survey was conducted. IQR=interquartile range.
| Country* | Year | Response rate (%) | Sample size† (n) | Sample median age (IQR), years† | Sample age range, years | Female (%) | Sample with diabetes† | Diabetes (%)‡ | Diagnosed (%)‡ |
|---|---|---|---|---|---|---|---|---|---|
| Low-income countries | |||||||||
| Bangladesh | 2011 | 95.0 | 7,305 | 48 (41–60) | 35–96 | 49.7 | 785 | 10.1 | 42.5 |
| Benin | 2015 | 98.6 | 4,041 | 39 (31–49) | 25–69 | 52.1 | 276 | 6.5 | 9.4 |
| Burkina Faso | 2013 | 98.7 | 3,945 | 37 (29–47) | 25–64 | 50.7 | 99 | 2.7 | 8.3 |
| Cambodia | 2010 | 96.3 | 5,026 | 43 (34–52) | 25–64 | 64.4 | 152 | 2.4 | 52.6 |
| Comoros | 2011 | 96.5 | 2,295 | 40 (32–50) | 25–64 | 73.9 | 98 | 4.3 | 57.1 |
| Eritrea | 2010 | 97.0 | 5,360 | 43 (33–55) | 25–74 | 70.9 | 203 | 3.7 | 53.9 |
| Liberia | 2011 | 87.1 | 1,543 | 35 (29–44) | 25–64 | 53.7 | 204 | 13.2 | 5.9 |
| Nepal | 2013 | 89.8 | 3,286 | 42 (35–54) | 25–69 | 68.3 | 192 | 5.4 | 44.4 |
| Rwanda | 2012 | 98.8 | 5,078 | 38 (31–48) | 25–64 | 62.2 | 85 | 1.6 | 14.8 |
| Tanzania§ | 2012 | 94.7 | 4,628 | 41 (33–50) | 25–64 | 52.2 | 142 | 2.8 | 39.1 |
| Togo | 2010 | 91.0 | 2,582 | 38 (30–47) | 25–64 | 50.5 | 82 | 3.3 | 21.7 |
| Uganda | 2014 | 92.2 | 2,633 | 38 (30–48) | 25–69 | 59.2 | 37 | 1.8 | 30.7 |
| Zanzibar§ | 2011 | 91.0 | 2,174 | 40 (32–50) | 25–64 | 61.3 | 95 | 3.6 | 37.2 |
| Lower-middle-income countries | |||||||||
| Bhutan | 2014 | 96.9 | 2,408 | 41 (33–51) | 25–69 | 60.1 | 72 | 2.5 | 38.7 |
| Eswatini | 2014 | 81.8 | 2,044 | 42 (32–54) | 25–71 | 67.2 | 154 | 6.7 | 42.6 |
| Georgia | 2016 | 75.7 | 2,973 | 53 (42–61) | 25–70 | 72.4 | 262 | 6.4 | 66.6 |
| India | 2015–16 | 96.0 | 491,512 | 35 (30–42) | 25–54 | 85.2 | 19,086 | 5.1 | 44.9 |
| Indonesia | 2014 | 83.0 | 5,350 | 48 (34–62) | 25–101 | 55.8 | 476 | 8.2 | 22.6 |
| Kenya | 2015 | 95.0 | 3,325 | 39 (31–50) | 25–69 | 59.5 | 105 | 2.4 | 29.7 |
| Kiribati | 2015 | 55.0 | 992 | 41 (32–51) | 25–69 | 55.6 | 209 | 19.9 | 38.0 |
| Kyrgyzstan | 2013 | N/A | 2,482 | 44 (35–53) | 25–64 | 63.1 | 153 | 5.4 | 47.4 |
| Laos | 2013 | 98.8 | 2,084 | 42 (33–50) | 25–65 | 59.9 | 127 | 5.7 | 54.8 |
| Lesotho | 2012 | 80.0 | 1,971 | 42 (32–54) | 25–64 | 65.8 | 82 | 2.8 | 41.8 |
| Moldova | 2013 | 83.5 | 3,371 | 51 (38–59) | 25–69 | 63.5 | 314 | 6.9 | 48.6 |
| Mongolia | 2013 | 97.4 | 1,866 | 40 (32–50) | 25–64 | 56.3 | 98 | 4.8 | 52.2 |
| Morocco | 2017 | 89.0 | 4,207 | 47 (37–59) | 25–100 | 65.0 | 663 | 13.6 | 53.0 |
| Myanmar | 2014 | 90.0 | 7,758 | 45 (36–54) | 25–64) | 65.1 | 614 | 6.4 | 47.0 |
| Samoa | 2013 | 64.0 | 1,306 | 43 (34–52) | 25–64 | 60.3 | 331 | 24.6 | 15.3 |
| Sao Tome and Principe | 2009 | 95.0 | 1,995 | 38 (30–48) | 25–64 | 56.1 | 59 | 3.0 | 44.6 |
| Solomon Islands | 2015 | 58.4 | 1,482 | 42 (34–51) | 25–71 | 54.3 | 94 | 5.4 | 12.2 |
| Sudan | 2015 | 88.0 | 5,311 | 40 (32–50) | 25–69 | 63.0 | 525 | 8.3 | 52.5 |
| Tajikistan | 2016 | 94.0 | 2,173 | 42 (33–52) | 25–70 | 58.5 | 202 | 5.5 | 29.4 |
| Timor Leste | 2014 | 96.3 | 2,021 | 43 (34–54) | 25–69 | 56.5 | 60 | 3.0 | 11.7 |
| Vanuatu | 2011 | 94.0 | 4,444 | 40 (32–50) | 25–64 | 49.1 | 470 | 9.7 | 18.4 |
| Vietnam | 2015 | 79.8 | 2,768 | 45 (37–55) | 25–69 | 57.2 | 108 | 3.1 | 53.1 |
| Zambia | 2017 | 74.3 | 2,565 | 40 (32–50) | 25–69 | 61.1 | 231 | 8.2 | 14.5 |
| Upper- middle- income countries | |||||||||
| Algeria | 2016 | 93.8 | 5,162 | 43 (35–53) | 25–69 | 54.9 | 683 | 11.6 | 64.1 |
| Azerbaijan | 2017 | 97.3 | 2,394 | 49 (37–57) | 25–69 | 59.6 | 263 | 8.2 | 55.9 |
| Belarus | 2016 | 87.1 | 4,423 | 49 (39–58) | 25–69 | 58.5 | 262 | 5.2 | 72.8 |
| Botswana | 2014 | 64.0 | 2,574 | 39 (31–51) | 25–69 | 68.9 | 117 | 3.8 | 40.3 |
| Chile | 2009–10 | 85.0 | 4,149 | 50 (38–63) | 25–100 | 60.5 | 540 | 11.8 | 66.3 |
| China | 2009 | 88.0 | 8,120 | 52 (42–62) | 25–99 | 53.2 | 643 | 7.9 | 36.1 |
| Costa Rica | 2010 | 87.8 | 2,433 | 50 (39–62) | 25–110 | 73.1 | 375 | 12.0 | 77.0 |
| Fiji | 2009 | 80.0 | 1,344 | 54 (47–63) | 40–90 | 57.1 | 581 | 43.2 | 33.9 |
| Guyana | 2016 | 66.7 | 702 | 45 (35–56) | 25–69 | 63.2 | 127 | 16.7 | 66.9 |
| Iran | 2016 | ~99 | 19,248 | 46 (35–58) | 25–100 | 53.5 | 1,740 | 9.6 | 77.6 |
| Iraq | 2015 | 98.8 | 3,187 | 43 (34–55) | 25–102 | 60.2 | 648 | 18.9 | 70.3 |
| Lebanon | 2017 | 65.9 | 1,106 | 49 (40–56) | 25–69 | 62.6 | 175 | 13.3 | 47.9 |
| Mexico | 2009–12 | 90.0 | 7,559 | 53 (45–64) | 25–99 | 54.8 | 2,539 | 35.3 | 44.2 |
| Namibia | 2013 | 96.9 | 3,244 | 46 (40–53) | 35–64 | 58.1 | 218 | 6.1 | 44.9 |
| Romania | 2015–16 | 69.1 | 1,774 | 50 (39–65) | 25–80 | 52.5 | 250 | 14.1 | 76.8 |
| Seychelles | 2013 | 73.0 | 1,240 | 47 (36–55) | 25–64 | 57.2 | 179 | 11.7 | 60.3 |
| South Africa | 2012 | 39.8 | 3,390 | 48 (36–60) | 25–97 | 65.2 | 575 | 13.3 | 46.6 |
| St. Vincent and the Grenadines | 2013 | 67.8 | 889 | 47 (37–55) | 25–70 | 60.7 | 115 | 11.1 | 79.4 |
| Tuvalu | 2015 | 76.0 | 860 | 47 (34–56) | 25–69 | 54.8 | 119 | 12.8 | 59.5 |
| Overall total | 680,102 | 52.7‡ | 37,094 | 9.0 | 43.9 | ||||
The sample includes nonpregnant participants that are ≥25 years of age with a non-missing diabetes biomarker.
Values account for survey design and are weighted equally by country in the overall total.
Zanzibar is a semi-autonomous region of the United Republic of Tanzania.
Coverage varied by treatment (Figure 1 and appendix p 56). Among pharmacological treatments, coverage of glucose-lowering medication was 50.5% (95% CI, 48.6–52.5); antihypertensive medication, 41.3% (95% CI, 39.3–43.3); and cholesterol-lowering medication, 6.3% (95% CI, 5.5–7.2). Among non-pharmacological treatments, coverage of diet counseling, 32.2% (95% CI, 30.7–33.7); exercise counseling, 28.2% (95% CI, 26.6–29.8); and weight-loss counseling, 31.5% (95% CI, 29.3–33.7). In the combined analysis, coverage of pharmacological treatment was 8.1% (95% CI, 7.1–9.1) and coverage of non-pharmacological treatment was 25.2% (95% CI, 23.7–26.7). Overall, 4.6% (95% CI, 3.9–5.4) of individuals with diabetes self-reported meeting need for all treatments defined in this study that were recommended for them in WHO PEN.
Figure 1: Diabetes treatment coverage in 55 low- and middle-income countries.
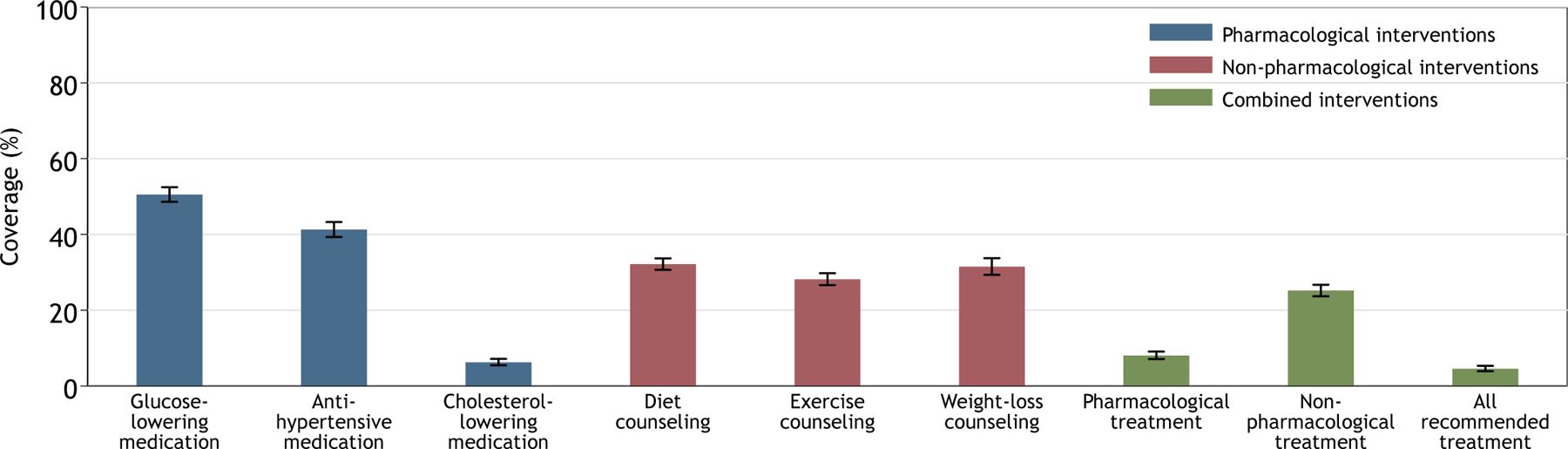
This figure displays coverage, or the proportion of eligible individuals receiving diabetes treatment, in 55 low-and middle-income countries. Each treatment is a core recommendation for people with type 2 diabetes in the 2020 World Health Organization Package of Essential Noncommunicable Disease Interventions. For the combined interventions, the denominator was all individuals who needed coverage for at least one treatment within the category; the numerator was the number of individuals self-reporting coverage for all treatments indicated for that individual within the category. For example, if an individual was defined to need glucose-lowering medication but not antihypertensive or cholesterol-lowering medication, the individual would be classified as having coverage for the pharmacological treatments if they self-reported use of the glucose-lowering medication (i.e., one out of only one indicated treatment). Conversely, if an individual was defined to need both glucose-lowering therapy and antihypertensive therapy, the individuals would not be classified as having coverage for the pharmacological treatments if the individual only self-reported use of the glucose-lowering medication (i.e., one out of two indicated interventions). Estimates account for clustering at the country level and equal weights by country. Error bars indicate 95% confidence intervals.
There were substantial differences in coverage by country stratifications of income and geographic region (Figure 2 and appendix p 56-59). Countries in higher income groups generally had higher coverage across all treatments. For example, coverage of glucose-lowering medication was 40.3% (95% CI, 36.4–44.3) in low-income countries, 45.1% (95% CI, 42.0–48.2) in lower-middle-income countries, and 64.1% (95% CI, 60.3–67.7) in upper-middle-income countries. Regionally, coverage was generally highest in Latin America and the Caribbean and lowest in Oceania followed by Sub-Saharan Africa. Across all income and geographic stratifications, coverage of glucose-lowering and hypertension medication was higher than coverage of non-pharmacological treatment of diet, exercise, and weight-loss counseling. Antihypertensive medication coverage also was higher than cholesterol-lowering medication coverage across income groups and regions.
Figure 2: Diabetes treatment coverage by country income group and geographic region.
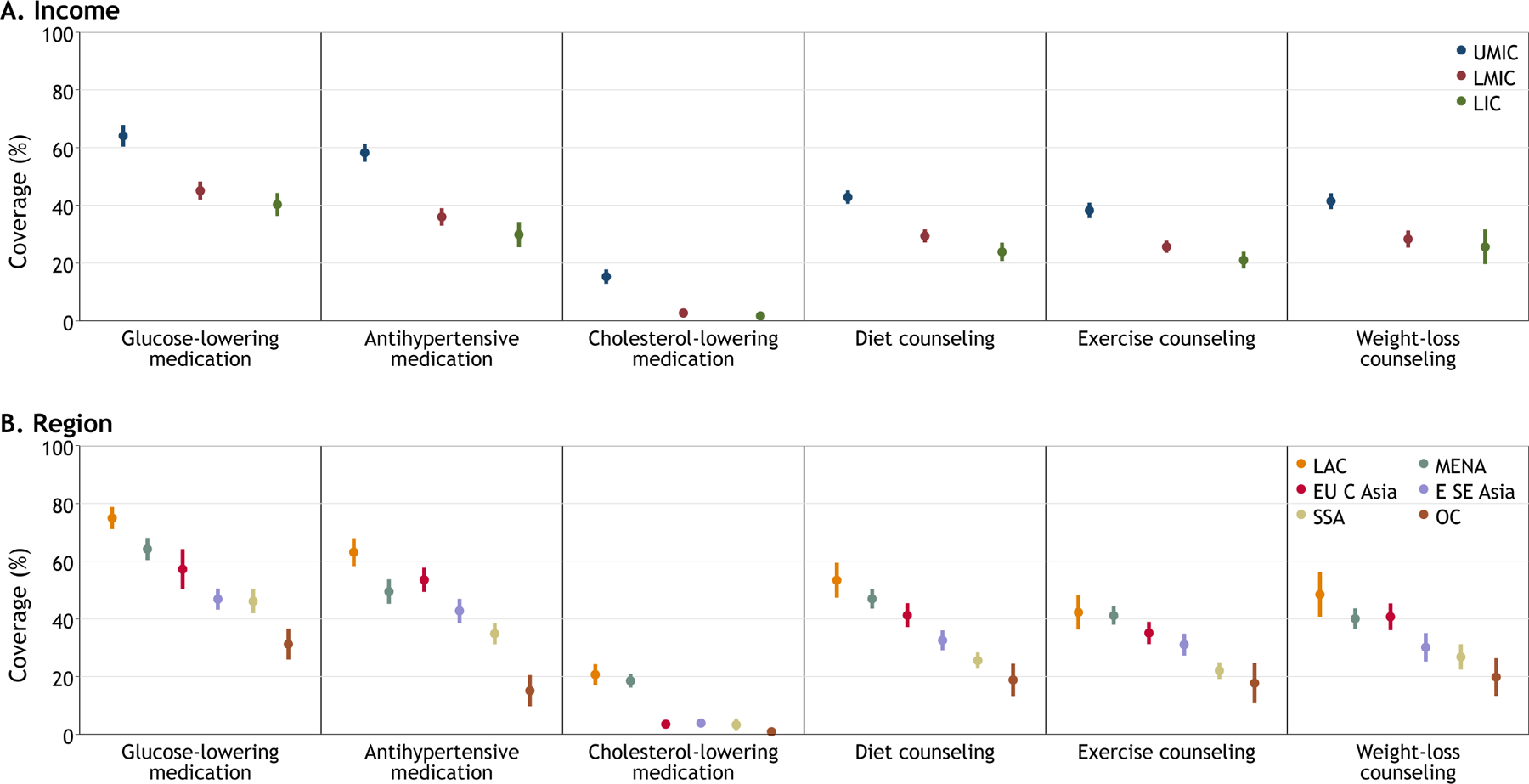
Data are percent with 95% confidence intervals accounting for clustering at the country level and equal weights by country. Income categories represent the World Bank’s classification in the year the survey was conducted. Geographical regions were categorized according to the NCD Risk Factor Collaboration.19 E SE Asia=East and Southeast Asia. EU C Asia=Europe and Central Asia. LAC=Latin America and Caribbean. LIC=low-income country. LMIC=lower-middle-income country. MENA=Middle East and North Africa. OC=Oceania. SSA=Sub-Saharan Africa. UMIC=upper-middle-income country.
The positive association between country income and coverage also was observed when countries’ per-capita GNI was included as a continuous variable among individuals ages 25–64 years (Figure 3 and appendix p 68-69). Countries that generally had higher diabetes treatment coverage than predicted based on income included Bangladesh, Costa Rica, Cambodia, Eritrea, Guyana, Iran, and Saint Vincent and the Grenadines. Countries that generally had lower diabetes treatment coverage than predicted based on income included Benin, Fiji, Indonesia, Rwanda, and Solomon Islands.
Figure 3: Diabetes treatment coverage by per capita gross national income among individuals 25–64 years of age.
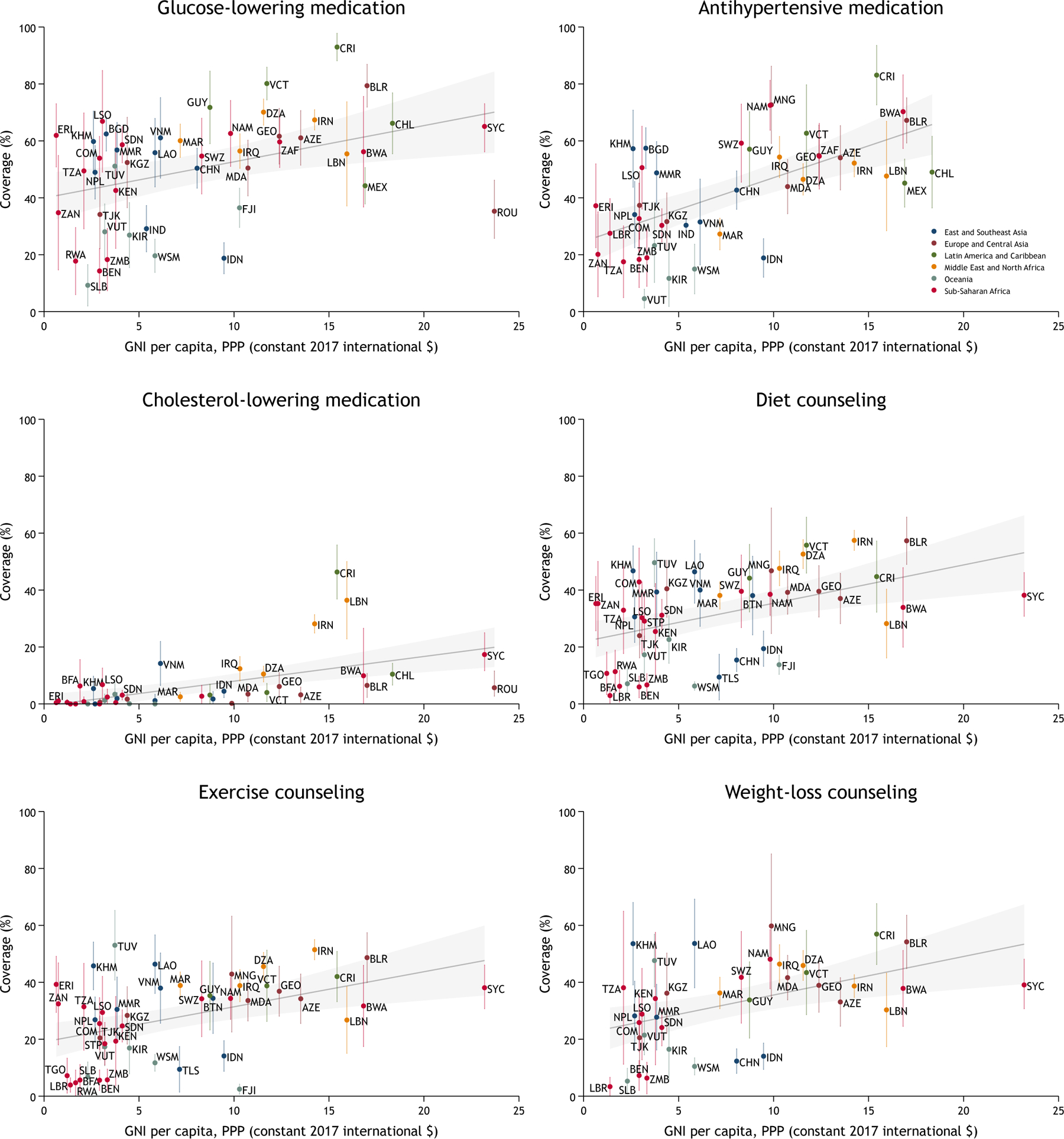
The gray shaded area represents the 95% linear prediction interval. The vertical bars represent 95% CIs around point estimates for a given country. The estimates account for weighting and survey design. Only countries with at least 50 individuals are included in this plot; results for all countries are depicted in appendix p 68-70. Gross national income (GNI) per capita is in constant 2017 international dollars as calculated by the World Bank for the year in which the survey was conducted. For countries with unavailable GNI data in the survey year, we used per-capita gross domestic product in constant 2017 international dollars. For Eritrea, we used per-capita GDP at current prices in 2011. For Zanzibar, we used estimates using constant 2015 international dollars as published by the Office of the Chief Government Statistician of Zanzibar. Some labels in the cholesterol-lowering medication plot are omitted due to space limitations. AZE=Azerbaijan, BEN=Benin, BFA=Burkina Faso, BGD=Bangladesh, BLR=Belarus, BTN=Bhutan, BWA=Botswana, CHL=Chile, CHN=China, COM=Comoros, CRI=Costa Rica, DZA=Algeria, ERI=Eritrea, FJI=Fiji, GEO=Georgia, GUY=Guyana, IDN=Indonesia, IND=India, IRN=Iran, IRQ=Iraq, KEN=Kenya, KGZ=Kyrgyzstan, KHM=Cambodia, KIR=Kiribati, LAO=Laos, LBN=Lebanon, LBR=Liberia, LSO=Lesotho, MAR=Morocco, MDA=Moldova, MEX=Mexico, MMR=Myanmar, MNG=Mongolia, NAM=Namibia, NPL=Nepal, ROU=Romania, RWA=Rwanda, SDN=Sudan, SLB=Solomon Islands, STP=Sao Tome and Principe, SWZ=Eswatini, SYC=Seychelles, TGO=Togo, TJK=Tajikistan, TLS=Timor Leste, TUV=Tuvalu, TZA=Tanzania, UGA=Uganda, VCT=St. Vincent & the Grenadines, VNM=Vietnam, VUT=Vanuatu, WSM=Samoa, ZAF=South Africa, ZAN=Zanzibar, ZMB=Zambia.
Predicted probabilities of coverage from the multivariable logistic regression models are shown in Table 3. See full model details in appendix p 60-64. Females had greater coverage than males for multiple treatments. There also tended to be a gradient of greater coverage with increasing age and BMI, higher levels of educational attainment, and, to a lesser extent, household wealth.
Table 3: Predicted probabilities of diabetes treatment coverage from multivariable logistic regression models.
Predicted probabilities of coverage with 95% confidence intervals are reported from multivariable logistic regression models. Predicted probabilities were generated as average adjusted predictions for categorical variables (sex, educational attainment, and wealth) and adjusted predictions at representative values for continuous variables (age and BMI). Wealth quintile was not available in n=7 surveys (appendix p 45). Each column represents a diabetes treatment that serves as the model’s dependent variable. The covariates in the model include all row variables and country-fixed effects. Illustrative values for age and body mass index are presented; these variables were included as continuous variables in the models using restricted cubic splines at knots of 5%, 27.5%, 50%, 72.5%, and 95%. Survey weighting and clustering at the country level were accounted for in the models. N/A=not applicable.
| Glucose-lowering medication | Antihypertensive medication | Cholesterol-lowering medication | Diet counseling | Exercise counseling | Weight-loss counseling | |
|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Sex | ||||||
| Male | 44.9 (41.9–47.9) | 34.4 (31.2–37.6) | 7.0 (5.3–8.7) | 31.3 (28.9–33.6) | 27.7 (25.3–25.3) | 28.8 (25.7–32.0) |
| Female | 52.2 (49.9–54.5) | 44.6 (41.8–47.3) | 6.7 (5.5–7.9) | 33.5 (31.4–35.6) | 29.8 (27.8–31.8) | 34.3 (31.5–37.1) |
| Age (years) | ||||||
| 30 | 24.0 (18.4–29.6) | 20.6 (13.4–27.8) | N/A | 13.1 (9.7–16.5) | 12.1 (8.8–15.5) | 17.2 (11.3–23.2) |
| 40 | 38.6 (34.8–42.4) | 29.4 (23.8–35.0) | 3.7 (1.5–5.8) | 25.5 (22.4–28.6) | 22.7 (19.6–25.7) | 27.9 (23.8–32.0) |
| 50 | 50.2 (46.7–53.6) | 38.2 (34.2–42.1) | 6.6 (5.0–8.3) | 34.3 (31.5–37.1) | 30.4 (27.6–33.3) | 32.7 (29.2–36.3) |
| 60 | 60.1 (56.6–63.7) | 49.1 (45.4–52.7) | 7.9 (6.0–9.8) | 41.6 (38.8–44.4) | 36.7 (34.0–39.4) | 38.8 (35.4–42.2) |
| Body mass index | ||||||
| 20 | 45.6 (41.2–50.0) | 30.7 (25.3–36.1) | 3.5 (1.8–5.2) | 23.0 (19.8–26.1) | 18.7 (15.7–21.7) | N/A |
| 25 | 48.7 (45.4–52.0) | 36.6 (33.4–39.9) | 6.5 (3.9–9.1) | 31.2 (28.3–34.0) | 28.3 (25.5–31.1) | 28.2 (19.5–36.9) |
| 30 | 50.3 (47.5–53.1) | 42.9 (39.8–46.1) | 6.9 (5.2–8.6) | 35.2 (32.5–37.9) | 30.9 (28.2–33.5) | 32.1 (28.2–36.0) |
| 35 | 51.7 (47.7–55.7) | 47.3 (43.0–51.6) | 8.2 (6.4–10.0) | 40.3 (36.4–44.2) | 35.6 (31.8–39.3) | 37.6 (33.4–41.7) |
| Education | ||||||
| None | 37.7 (32.9–42.6) | 34.6 (30.4–38.7) | 6.6 (4.5–8.8) | 25.0 (21.4–28.7) | 19.8 (16.2–23.4) | 23.8 (18.5–29.1) |
| Primary | 49.0 (45.8–52.2) | 40.9 (37.2–44.6) | 7.2 (5.8–8.6) | 33.3 (30.5–36.0) | 28.2 (25.5–30.9) | 32.6 (29.0–36.3) |
| Secondary or more | 53.0 (49.9–56.0) | 42.4 (39.0–45.7) | 6.6 (4.6–8.5) | 34.7 (32.0–37.5) | 32.9 (30.1–35.7) | 34.6 (31.2–37.9) |
| Wealth quintile | ||||||
| 1 (poorest) | 47.8 (44.0–51.7) | 40.4 (36.1–44.8) | 7.2 (5.3–9.1) | 29.7 (26.2–33.2) | 25.5 (22.0–28.9) | 27.6 (23.1–32.1) |
| 2 | 44.9 (41.2–48.6) | 39.7 (35.2–44.1) | 6.5 (4.0–9.1) | 27.8 (24.7–30.9) | 25.3 (22.0–28.6) | 26.9 (22.8–31.1) |
| 3 | 45.6 (41.6–49.6) | 41.6 (36.7–46.4) | 5.1 (3.8–6.3) | 29.4 (26.2–32.6) | 27.0 (23.8–30.1) | 30.1 (25.9–34.2) |
| 4 | 50.8 (47.2–54.4) | 40.4 (36.3–44.5) | 7.2 (5.3–9.1) | 35.7 (32.0–39.4) | 30.2 (26.4–34.0) | 33.2 (28.6–37.9) |
| 5 (richest) | 54.5 (50.3–58.7) | 39.6 (35.1–44.2) | 8.5 (5.1–12.0) | 38.7 (34.9–42.5) | 35.3 (31.7–38.9) | 39.7 (35.0–44.4) |
In the sensitivity analysis restricting the sample to individuals reporting a prior diabetes diagnosis (appendix p 65), coverage of glucose-lowering medication was 85.0% (95% CI, 82.3–87.4); antihypertensive medication, 57.0% (95% CI, 53.9–60.0); cholesterol-lowering medication, 9.2% (95% CI, 7.7–10.9); diet counseling, 73.9% (95% CI, 71.4–76.3); exercise counseling, 65.3% (95% CI, 62.8–67.8); and weight-loss counseling, 62.8% (95% CI, 59.3–66.1). In this scenario, the combined coverage of pharmacological treatment was 14.3% (95% CI, 12.2–16.8); non-pharmacological treatment, 56.2% (95% CI, 53.6–58.8); and all recommended treatments, 10.1% (95% CI, 8.2–12.5). In the sensitivity analysis weighting each country in proportion to its 2015 population size (appendix p 66), the overall coverage was similar for antihypertensive medication (40.5% [95% CI, 38.0–43.1]), cholesterol-lowering medication (7.9% [95% CI, 6.8–9.1), and exercise counseling (27.8% [95% CI, 24.4–31.5]); however, coverage was lower for glucose-lowering medication (45.6% [95% CI, 42.0–49.2]), diet counseling (23.5% [95% CI, 21.2–26.0]), and weight-loss counseling (19.0% [95% CI, 16.3–21.9]). Results from the sensitivity analysis restricted to the sample of individuals 25–64 years of age is depicted in Figure 3 and appendix p 67-70. In the sensitivity analysis among people with diabetes who had a 10-year predicted CVD risk ≥20% (appendix p 71), coverage of cholesterol-lowering medication was 7.6% (95% CI, 5.4–10.6). Finally, results from the sensitivity analysis with multivariable logistic regression models using random effects at the country level were generally consistent with the primary analysis (appendix p 72).
DISCUSSION
In this pooled analysis of nationally representative, individual-level data from 55 LMICs, we found that fewer than one in ten people with diabetes achieved a composite indicator of coverage for up to six clinical treatments recommended in WHO guidelines. Moreover, less than 15% of people with previously diagnosed diabetes had achieved the composite indicator. Coverage of glucose-lowering medication was modestly higher than antihypertensive medication and substantially higher than cholesterol-lowering medication. Among all people with diabetes, about one in three people self-reported receiving recommended non-pharmacological treatment in the form of counseling about diet, exercise, or weight loss.
Additionally, we found that countries in higher income categories tended to achieve greater coverage across treatments. We also identified countries that generally had higher coverage than would be predicted based on per-capita income. Costa Rica emerged as an example of one such well-performing country, a result that has been attributed to the country’s commitment to universal health coverage.21 Other countries that outperformed predicted coverage based on per-capita income included Bangladesh, Cambodia, Eritrea, Guyana, Iran, and Saint Vincent and the Grenadines. When countries were analyzed by geographic region, countries in Oceania tended to have the lowest coverage across treatments despite having the highest overall prevalence of diabetes. Prior research on diabetes among people of this region suggests that high diabetes prevalence and limited implementation of diabetes care results from complex structural forces.22 The region of Latin America and the Caribbean had the second-to-highest diabetes prevalence, but this region also consistently had among the highest levels of coverage across treatments.
At the individual level, we found that women had greater coverage across multiple treatments. This finding is consistent with the broad global literature detailing lower utilization of primary health care services among men in low-resource settings.23 Greater coverage also was associated with higher age, BMI, educational attainment, and household wealth. The results for age and BMI suggest that health systems in LMICs are reaching more people with diabetes who have traditional risk factors such as older age and high BMI. These findings are critical given that there is a high proportion of individuals with diabetes in LMICs who are normal weight according to clinically defined BMI categories,24 and adequate diabetes treatment in a young person may confer health and economic benefits throughout the lifespan.
A strength of our study is that, in our primary analysis, we define diabetes using an individual’s diabetes biomarker or use of a glucose-lowering medication, rather than limiting the analysis only to individuals reporting a prior diabetes diagnosis. Given substantial differences in the proportion of people diagnosed between countries, we believe that our approach is the most valid method to compare performance of health systems in delivering diabetes treatment to the overall population in need, which was our study’s main objective. In a sensitivity analysis, we restrict the sample to those reporting a diabetes diagnosis and find that large differences persist in diabetes treatment coverage among the three pharmacological treatments and also between pharmacological versus non-pharmacological treatments. As a high-income country comparison among people previously diagnosed with diabetes, in the 2007–2012 U.S. National Health and Nutrition Examination (NHANES) survey, the proportion of eligible adults with diabetes who self-reported use of an antihypertensive medication for hypertension and statin use for primary CVD prevention was approximately 90% and 52%, respectively,25 rates which are substantially higher than the results from the LMICs included in our study. However, the prevalence of self-reported exercise, diet, and weight-loss counseling among people with diagnosed diabetes in the 2011–2016 U.S. NHANES survey was approximately 70%,26 rates which are relatively similar to our results. In a pooled study of health examination surveys from 12 high-income countries, overall treatment of hypertension (among all hypertensive individuals) ranged from 40% to 80%, while we found that 57.0% (95% CI, 53.9–60.0) of people with diabetes were treated in our sample of countries.27 In the 2013–2017 Japan National Health and Nutrition Surveys, coverage for glucose-lowering and antihypertensive therapy among those in need of treatment was 79.9% and 46.3%, respectively.28 The Prospective Urban Rural Epidemiology (PURE) study in 21 countries also has shown markedly higher diabetes medication use and lower CVD mortality among people with diabetes in high-income countries compared with LMICs.3
Such comparisons between our results and previously published reports from high-income countries should be made cautiously, as there are likely to be differences in methods and definitions among surveys. Nevertheless, our findings suggest that antihypertensive and cholesterol-lowering medication are areas of diabetes treatment in which there is a sizeable gap between health systems in LMICs versus those in high-income countries. Randomized clinical trials demonstrate the high degree of absolute clinical benefit in addressing hypertension and elevated cholesterol among people with diabetes, as reflected in more favorable numbers needed to treat for antihypertensive and cholesterol-lowering medications (i.e., statins) compared with intensive glucose-lowering therapy.29 Non-pharmacological interventions for diabetes, while widely recommended, have less evidence for improvement in long-term outcomes.11 Taken in this context, our results suggest that scaling-up pharmacological treatment of CVD risk factors such as hypertension and elevated cholesterol are priorities for the management of diabetes in LMICs. While modeling studies30 and consensus reports such as Disease Control Priorities, 3rd edition31 have pointed to the cost-effectiveness of this approach, implementation will require substantial investments in health systems in LMICs. Global initiatives such as the WHO HEARTS Technical Package7—which integrates management of diabetes within a CVD prevention package in primary health care—and the forthcoming WHO Global Diabetes Compact32 represent important opportunities to address these implementation and economic challenges. In the recently published Lancet Commission on diabetes, global experts highlighted the urgent need to scale-up treatment of cardiometabolic risk factors among people with diabetes through a multifaceted health systems strengthening approach that includes deployment of non-physician health workers (i.e., task sharing) within a team-based approach, ensuring access to affordable medications including insulin, and strengthened data systems to measure health system performance.15 While there is also a need to develop prospective, longitudinal data sources such as patient registries in LMICs,15 our study also demonstrates the power of population-based surveys such as STEPS to benchmark health system performance for diabetes. Analysis of repeated surveys from the same country over time, though beyond the scope of the present work, would also aid in understanding temporal trends in health systems’ ability to deliver diabetes treatment within and across countries.
To our knowledge, this study is the most comprehensive assessment of diabetes treatment using nationally representative data in LMICs of diverse income groups and geographic regions. Previous pooled studies of diabetes treatment in LMICs include the PURE study,3 an analysis of diabetes and CVD risk factors in a total of seven countries including four LMICs,33 and our group’s analysis of diabetes care cascades in 28 LMICs.14 The PURE study also reported low use of glucose-lowering, antihypertensive, and cholesterol-lowering medications in LMICs, but the study was limited by non-nationally representative samples, inclusion of fewer than 20 LMICs, and, to our knowledge, no estimates for non-pharmacological diabetes interventions.3 Our group’s prior work in 28 LMICs demonstrated substantial gaps in the cascade of glycemic care, especially at the step of diagnosis, but this study did not explore coverage of antihypertensive or cholesterol-lowering medication.14 The present study makes a significant contribution to the global diabetes literature by evaluating coverage of comprehensive diabetes treatment in a much larger sample of nationally representative country surveys in LMICs than has previously been available.
Our study has limitations. First, we define diabetes diagnosis based on a single diabetes biomarker, many of which are capillary glucose measurements. Single measurements of capillary glucose are common in high-quality pooled diabetes surveys19,34 though there are concerns about diagnostic misclassification and the need for conversion to plasma glucose.35 In our study, misclassification may lead to both underdiagnosis and overdiagnosis. Underdiagnosis may occur as a single fasting glucose may fail to capture 30% or more of people with undiagnosed diabetes who would be detected using an oral glucose tolerance test.36–38 Conversely, overdiagnosis may occur as the short-term variability of a single fasting glucose may inflate diagnoses by as much as 30%.39,40 Second, variations in diabetes clinical guidelines between countries may account for some observed differences.41 Additionally, in clinical practice, the decision to start or stop a pharmacological treatment may be related to patients’ observed responses to non-pharmacological treatment. For example, in patients with modestly elevated blood glucose, clinicians may appropriately defer initiating glucose-lowering treatment while first focusing on lifestyle changes through counseling on diet, exercise, and weight loss. While acknowledging these nuances, we justify our outcomes as they are based on population-monitoring indicators and clinical treatment recommendations in WHO PEN, which is a widely cited global reference standard for NCD management in LMICs.6 Third, differences among surveys regarding the year the survey was conducted, diabetes biomarker, age sample, and how surveys implemented the same question may account for some of the observed variation and affect cross-country comparisons. Despite these differences, to our knowledge, the included surveys represent the best available population-level data on diabetes treatment in LMICs. We include extensive information on the underlying surveys in our Appendices, and we attempt to investigate these differences through a suite of sensitivity analyses. Fourth, as we define coverage using self-reported measures, our estimates may be impacted by recall bias or the potential for self-reports to overestimate coverage.42 Fifth, the underlying surveys had incomplete inclusion of each outcome, and, in particular, there were a lack of non-pharmacological data from several large countries such as Bangladesh, India, and Mexico. Similarly, due to unavailability of the relevant variables, we were unable to pool data on the coverage of smoking cessation counseling; this is a limitation as smoking is a major contributor to CVD risk among people with diabetes. Sixth, we do not consider the topic of diabetes prevention or the role of new agents such as sodium glucose co-transporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, which reduce mortality among diabetes patients at high CVD risk.43 These are active priority areas for future research within our group.
A final limitation is that our study focuses on treatment coverage and does not assess the quality of coverage or the adequacy of risk factor control. The Lancet Global Health Commission on High Quality Health Systems advocates for the use of “effective coverage,” a quality-corrected coverage metric, to benchmark health system performance.44 In contrast to solely assessing treatment coverage conditional on need, as in our study, effective coverage also accounts for the magnitude of health gain experienced by individual receiving treatment.45 In cross-sectional data such as that used in this study, it is very challenging to estimate the health gains associated with treatment. Given these challenges, we define our coverage outcome to mirror the population-level monitoring indicators recommended in WHO PEN, which are in turn based on existing clinical evidence.6 In future research, we plan to provide estimates of effective coverage of diabetes treatment.
In conclusion, we found that fewer than one in ten people with diabetes in LMICs receive coverage of guideline-based comprehensive diabetes treatment. Our findings suggest that delivering treatment not only to lower glucose but also to manage CVD risk factors such as hypertension and elevated cholesterol among people with diabetes are urgent global priorities. Policy efforts to achieve Sustainable Development Goal 3.4 should focus on filling these gaps in global diabetes care.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed on January 5, 2021 without language or date restrictions using the search terms of “diabetes” and (“low- and middle-income countries” or “developing countries”) and (“met need” or “treatment” or “coverage”) in the title or abstract. We identified two large studies assessing levels of diabetes treatment using individual data from multiple low- and middle-income countries (LMICs). One study reported estimates of glycemic care (proportion tested, diagnosed, treated, controlled) among individuals with diabetes in 28 LMICs but did not explore other components of comprehensive diabetes care such as medication treatment for hypertension or elevated cholesterol. A second study, the Prospective Urban Rural Epidemiology (PURE) study, reported individual-level data on use of diabetes, hypertension, and cardiovascular disease medicines in low-and-middle-income countries. However, the PURE study is not nationally representative, includes fewer than 20 LMICs, and, to our knowledge, has not assessed utilization of non-pharmacological interventions for diabetes.
Added value of this study
To our knowledge, this study is the largest assessment of comprehensive diabetes treatment using individual-level data from nationally representative samples of adults across LMICs of diverse income groups and geographic regions. This study makes three valuable additions to the existing literature. First, we found that fewer than one in ten people with diabetes in LMICs receive comprehensive diabetes treatment as recommended by the World Health Organization (WHO). Our findings are essential for policymakers as we identify pharmacological treatment of hypertension and elevated cholesterol as key drivers of low self-reported treatment. Second, we make cross-country estimates of diabetes treatment that may be used by health systems in LMICs to benchmark current and future performance. Third, we report individual characteristics such as young age and lower BMI that are associated with low coverage of diabetes treatment; these individual-level findings can be used by health systems to direct care to underserved groups.
Implications of all the available evidence
While there are differences in the proportion of people receiving diabetes treatment both between and within countries, overall, there is need to scale-up comprehensive diabetes treatment in LMICs. Our findings suggest that improving access to comprehensive treatment not only to lower glucose but also to address cardiovascular disease risk factors such as hypertension and elevated cholesterol are global diabetes priorities. Global initiatives such as the WHO HEARTS Technical Package and Global Diabetes Compact represent important opportunities to address the implementation and economic challenges of this approach.
Funding
DF was supported by the National Clinician Scholars Program at the University of Michigan Institute for Healthcare Policy & Innovation. JAS was supported by Grant Number T32DK007028 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and by Grant Number 5KL2TR002542–03 (Harvard Catalyst). PG was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR003143. MH was supported by Grant Number P30DK092926 (MCDTR) from the National Institute of Diabetes and Digestive and Kidney Diseases. JMG was supported by grant K23 DK125162 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The contents of this research are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Declaration of interests
D.J.W. reports serving on a data-monitoring committee for Novo Nordisk.
Data sharing statement
This study includes individual-level data from 55 countries. Data are publicly available for 47 of these countries. For data that are not publicly accessible and for which we have arranged specific data-use agreements, we are unable to share these data given the terms of our agreements. Appendix p 35 provides a complete list of web addresses and country-specific contacts regarding data access.
Contributor Information
David Flood, Division of Hospital Medicine, Department of Internal Medicine, National Clinician Scholars Program, University of Michigan, Ann Arbor, MI, USA; Center for Indigenous Health Research, Wuqu’ Kawoq; Tecpán, Guatemala; Research Center for the Prevention of Chronic Diseases, Institute of Nutrition of Central America and Panama, Guatemala City, Guatemala.
Jacqueline A. Seiglie, Diabetes Unit, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA
Matthew Dunn, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Scott Tschida, Center for Indigenous Health Research, Wuqu’ Kawoq; Tecpán, Guatemala;.
Michaela Theilmann, Institute of Global Health, Heidelberg University, Heidelberg, Germany.
Maja E. Marcus, Department of Economics and Centre for Modern Indian Studies, University of Goettingen, Göttingen, Germany
Garry Brian, The Fred Hollows Foundation New Zealand, Auckland, New Zealand.
Bolormaa Norov, National Center for Public Health, Ulaanbaatar, Mongolia.
Mary T. Mayige, National Institute for Medical Research, Dar es Salaam, Tanzania
Mongal Singh Gurung, Health Research and Epidemiology Unit, Ministry of Health, Bhutan.
Krishna K. Aryal, Monitoring Evaluation and Operational Research Project, Abt Associates, Kathmandu, Nepal
Demetre Labadarios, Faculty of Medicine and Health Sciences, Stellenbosch University, Stellenbosch, South Africa.
Maria Dorobantu, University of Medicine and Pharmacy, Carol Davila, Bucharest, Romania.
Bahendeka K. Silver, St. Francis Hospital, Nsambya, Kampala, Uganda
Pascal Bovet, Ministry of Health, Victoria, Republic of Seychelles; University Center for Primary Care and Public Health, Lausanne, Switzerland.
Jutta M. Adelin Jorgensen, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
David Guwatudde, Department of Epidemiology and Biostatistics, School of Public Health, Makerere University, Kampala, Uganda.
Corine Houehanou, Laboratory of Epidemiology of Chronic and Neurological Diseases, Faculty of Health Sciences, University of Abomey-Calavi, Benin.
Glennis Andall-Brereton, Caribbean Public Health Agency, Port of Spain, Trinidad and Tobago.
Sarah Quesnel-Crooks, Non-Communicable Disease Department, National Center for Disease Control and Public Health, Tbilisi, Georgia.
Lela Sturua, Non-Communicable Disease Department, National Center for Disease Control and Public Health, Tbilisi, Georgia; Petre Shotadze Tbilisi Medical Academy, Tbilisi, Georgia.
Farshad Farzadfar, Non-Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
Sahar Saeedi Moghaddam, Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
Rifat Atun, Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA; Department of Global Health and Social Medicine, Harvard Medical School, Harvard University, Boston, MA, USA.
Sebastian Vollmer, Department of Economics and Centre for Modern Indian Studies, University of Goettingen, Göttingen, Germany.
Till W. Bärnighausen, Institute of Global Health, Heidelberg University, Heidelberg, Germany; Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA; Africa Health Research Institute, Somkhele, South Africa
Justine I. Davies, MRC/Wits Rural Public Health and Health Transitions Research Unit, School of Public Health, University of Witwatersrand, Johannesburg, South Africa; Institute of Applied Health Research, University of Birmingham, Birmingham, UK; Centre for Global Surgery, Department of Global Health, Stellenbosch University, Cape Town
Deborah J. Wexler, Diabetes Unit, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA
Pascal Geldsetzer, Institute of Global Health, Heidelberg University, Heidelberg, Germany; Division of Primary Care and Population Health, Department of Medicine, Stanford University, Stanford, CA, USA.
Peter Rohloff, Center for Indigenous Health Research, Wuqu’ Kawoq; Tecpán, Guatemala; Division of Global Health Equity, Brigham and Women’s Hospital; Boston MA, USA.
Manuel Ramírez-Zea, Research Center for the Prevention of Chronic Diseases, Institute of Nutrition of Central America and Panama, Guatemala City, Guatemala.
Michele Heisler, Department of Internal Medicine, School of Medicine, University of Michigan, Ann Arbor, MI, USA; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI, USA; Veterans Affairs Ann Arbor Center for Clinical Management Research, Ann Arbor, MI, USA.
Jennifer Manne-Goehler, Division of Infectious Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
REFERENCES
- 1.Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 2020; 396(10258): 1223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas, 9th edition: International Diabetes Federation, 2019. [Google Scholar]
- 3.Anjana RM, Mohan V, Rangarajan S, et al. Contrasting Associations Between Diabetes and Cardiovascular Mortality Rates in Low-, Middle-, and High-Income Countries: Cohort Study Data From 143,567 Individuals in 21 Countries in the PURE Study. Diabetes Care 2020. [DOI] [PMC free article] [PubMed]
- 4.Kruk ME, Gage AD, Joseph NT, Danaei G, García-Saisó S, Salomon JA. Mortality due to low-quality health systems in the universal health coverage era: a systematic analysis of amenable deaths in 137 countries. The Lancet 2018; 392(10160): 2203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCD Countdown 2030: pathways to achieving Sustainable Development Goal target 3.4. The Lancet 2020; 396(10255): 918–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. WHO package of essential noncommunicable (PEN) disease interventions for primary health care. Geneva: World Health Organization, 2020. [Google Scholar]
- 7.WHO. Diagnosis and management of type 2 diabetes (HEARTS-D). Geneva: World Health Organization, 2020. [Google Scholar]
- 8.Rawshani A, Rawshani A, Franzen S, et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2018; 379(7): 633–44. [DOI] [PubMed] [Google Scholar]
- 9.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348(5): 383–93. [DOI] [PubMed] [Google Scholar]
- 10.Ali MK, Siegel KR, Chandrasekar E, et al. Diabetes: An Update on the Pandemic and Potential Solutions. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, eds. Cardiovascular, Respiratory, and Related Disorders. Washington (DC): World Bank; 2017. [PubMed] [Google Scholar]
- 11.Look Ahead Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369(2): 145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flood D, Hane J, Dunn M, et al. Health system interventions for adults with type 2 diabetes in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med 2020; 17(11): e1003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivashankar R, Kirk K, Kim WC, et al. Quality of diabetes care in low- and middle-income Asian and Middle Eastern countries (1993–2012): 20-year systematic review. Diabetes Res Clin Pract 2015; 107(2): 203–23. [DOI] [PubMed] [Google Scholar]
- 14.Manne-Goehler J, Geldsetzer P, Agoudavi K, et al. Health system performance for people with diabetes in 28 low- and middle-income countries: A cross-sectional study of nationally representative surveys. PLoS Med 2019; 16(3): e1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancet Commission. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. 2020. [DOI] [PubMed]
- 16.Seiglie JA, Marcus ME, Ebert C, et al. Diabetes Prevalence and Its Relationship With Education, Wealth, and BMI in 29 Low- and Middle-Income Countries. Diabetes Care 2020; 43(4): 767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2011; 57(6): e1–e47. [DOI] [PubMed] [Google Scholar]
- 18.Wei N, Zheng H, Nathan DM. Empirically establishing blood glucose targets to achieve HbA1c goals. Diabetes Care 2014; 37(4): 1048–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risk Factor Collaboration N. C. D. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016; 387(10027): 1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaptoge S, Pennells L, De Bacquer D, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. The Lancet Global Health 2019. [DOI] [PMC free article] [PubMed]
- 21.Pesec M, Ratcliffe HL, Karlage A, Hirschhorn LR, Gawande A, Bitton A. Primary Health Care That Works: The Costa Rican Experience. Health Aff (Millwood) 2017; 36(3): 531–8. [DOI] [PubMed] [Google Scholar]
- 22.Foliaki S, Pearce N. Prevention and control of diabetes in Pacific people. BMJ 2003; 327(7412): 437–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker P, Shand T. Men’s health: time for a new approach to policy and practice? J Glob Health 2017; 7(1): 010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gujral UP, Weber MB, Staimez LR, Narayan KMV. Diabetes Among Non-Overweight Individuals: an Emerging Public Health Challenge. Curr Diab Rep 2018; 18(8): 60. [DOI] [PubMed] [Google Scholar]
- 25.Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014; 161(10): 681–9. [DOI] [PubMed] [Google Scholar]
- 26.Grabovac I, Smith L, Stefanac S, et al. Health Care Providers’ Advice on Lifestyle Modification in the US Population: Results from the NHANES 2011–2016. Am J Med 2019; 132(4): 489–97 e1. [DOI] [PubMed] [Google Scholar]
- 27.Zhou B, Danaei G, Stevens GA, et al. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. The Lancet 2019; 394(10199): 639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda N, Nishi N, Sugiyama T, Noda H, Noda M. Effective coverage of medical treatment for hypertension, diabetes and dyslipidaemia in Japan: An analysis of National Health and Nutrition Surveys 2003–2017. J Health Serv Res Policy 2020: 1355819620949574. [DOI] [PubMed] [Google Scholar]
- 29.Yudkin JS, Richter B, Gale EA. Intensified glucose lowering in type 2 diabetes: time for a reappraisal. Diabetologia 2010; 53(10): 2079–85. [DOI] [PubMed] [Google Scholar]
- 30.Basu S, Wagner RG, Sewpaul R, Reddy P, Davies J. Implications of scaling up cardiovascular disease treatment in South Africa: a microsimulation and cost-effectiveness analysis. Lancet Glob Health 2019; 7(2): e270–e80. [DOI] [PubMed] [Google Scholar]
- 31.Prabhakaran D, Anand S, Watkins D, et al. Cardiovascular, respiratory, and related disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 2018; 391(10126): 1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO Global Diabetes Compact. 2020. https://www.who.int/docs/default-source/world-diabetes-day/global-diabetes-compact-final.pdf.
- 33.Gakidou E, Mallinger L, Abbott-Klafter J, et al. Management of diabetes and associated cardiovascular risk factors in seven countries: a comparison of data from national health examination surveys. Bull World Health Organ 2011; 89(3): 172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emerging Risk Factors C, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375(9733): 2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stauffer F, Viswanathan B, Jean M, Kinabo P, Bovet P. Comparison between capillary glucose measured with a Contour glucometer and plasma glucose in a population survey. LaboratoriumsMedizin 2016; 40(2). [Google Scholar]
- 36.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. 2006.
- 37.Zhou X, Pang Z, Gao W, et al. Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care 2010; 33(3): 545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danaei G, Fahimi S, Lu Y, et al. Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population-based studies with 331 288 participants. The Lancet Diabetes & Endocrinology 2015; 3(8): 624–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007; 167(14): 1545–51. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt MI, Bracco P, Canhada S, et al. Regression to the Mean Contributes to the Apparent Improvement in Glycemia 3.8 Years After Screening: The ELSA-Brasil Study. Diabetes Care 2021; 44(1): 81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owolabi MO, Yaria JO, Daivadanam M, et al. Gaps in Guidelines for the Management of Diabetes in Low- and Middle-Income Versus High-Income Countries-A Systematic Review. Diabetes Care 2018; 41(5): 1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015; 5(4): 470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann Intern Med 2020; 173(4): 278–86. [DOI] [PubMed] [Google Scholar]
- 44.Kruk ME, Gage AD, Arsenault C, et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. The Lancet Global Health 2018; 6(11): e1196–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng M, Fullman N, Dieleman JL, Flaxman AD, Murray CJ, Lim SS. Effective coverage: a metric for monitoring Universal Health Coverage. PLoS Med 2014; 11(9): e1001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


