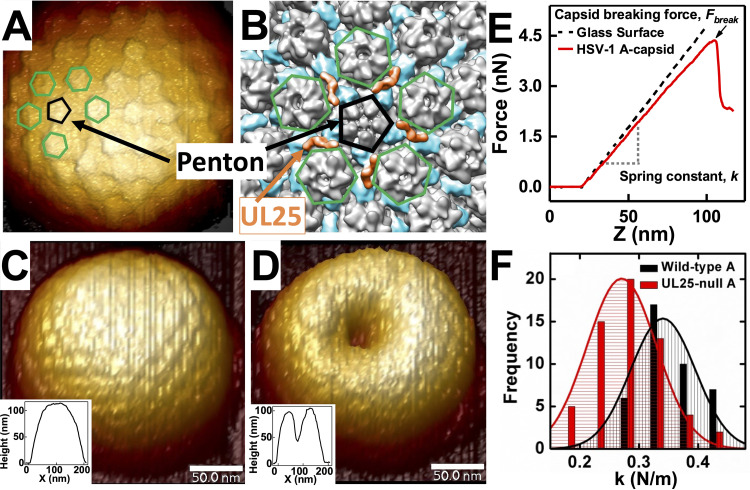
FIG 4.
(A) Cryo-EM reconstruction (EMD-1354 (54)) is colored to indicate the positions of UL25 (orange) in relation to the penton (black pentagon outline). UL25 connects capsomers by binding with the penton and the underlying triplexes (blue on reconstruction). Neighboring hexons are outlined in green. (B) A high-resolution AFM image of HSV-1 A-capsid with a penton (black) and its five neighboring hexons (green) are indicated. (C) AFM image of intact HSV-1 A-capsid in liquid. The inset shows a topographical profile. (D) AFM image and topographical profile of the same HSV-1 A-capsid after breaking with nano-indentation. (E) Force-distance (FZ) curves for glass substrate and HSV-1 A-capsid. Capsid breaking is observed as a drop in the force curve. FZ curve allows direct measurement of Fbreak and k values. The k values are quantified by fitting Gaussian functions to histograms of multiple measurements of unique capsids. (F) Representative histograms of spring constants, k, for HSV-1 WT A-capsid versus UL25-null A-capsid showing a weakening of the A-capsid with deleted UL25.