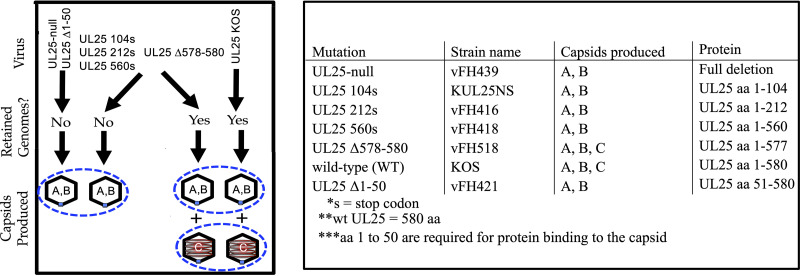
FIG 6.
Many of the characterized UL25 mutations can be divided into three types. The first represents mutations that inhibit UL25 binding to capsids and result in a loss of infectivity. These mutations typically map to the N-terminal domain of UL25, which is essential for capsid interaction. Because these mutants do not bind capsids, their stability would be identical to UL25-null capsids. The second type of mutants binds capsids but does not retain packaged genomes. The third type consisted of a mutant that was missing the last three amino acids of UL25. This UL25 mutant Δ578-580 (UL25 aa 1 to 577) binds to capsids, and the capsids retained packaged genomes forming C-capsids but failed to produce infectious viruses. All three types of mutants were used to investigate the role of UL25 in capsid reinforcement. Coils in the C-capsids represent packaged DNA while A- and B-capsids are empty. Table 1 shows the list of HSV-1 UL25 mutants analyzed with AFM nano-indentation assay.