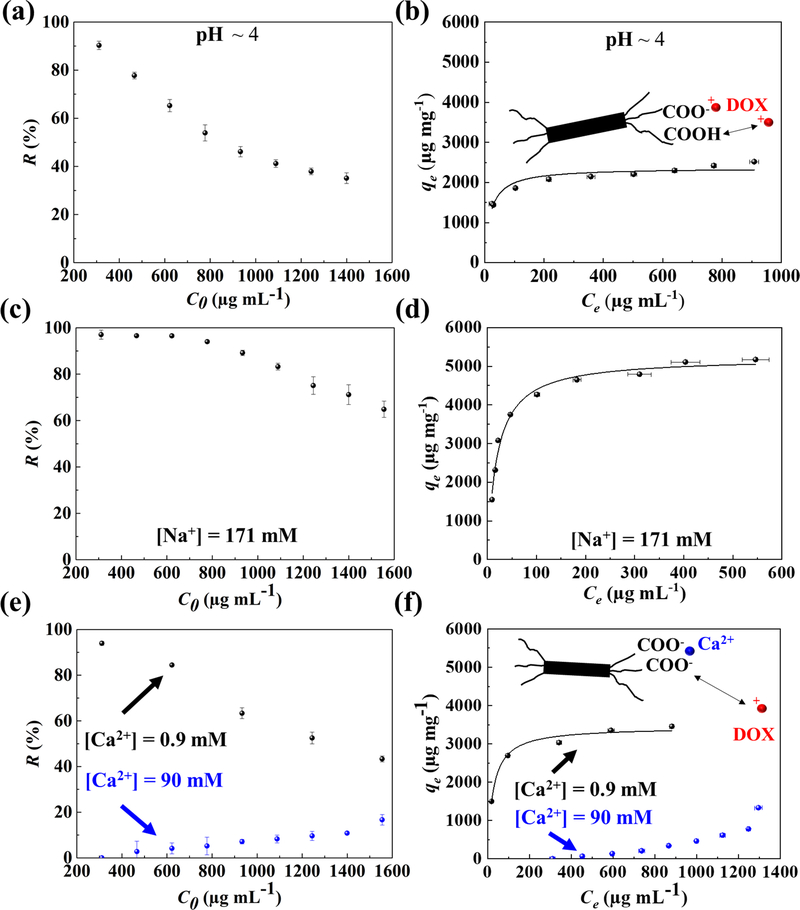
Fig. 3. Effects of pH and ionic strength on ENCC-mediated DOX capture.
(a) DOX removal percentage versus initial DOX concentration, and (b) DOX capture capacity of ENCC versus equilibrium DOX concentration at pH ~ 4. Decreasing pH to 4 resulted in the protonation of carboxylic acid groups, which decreased the available DOX binding sites on ENCC, decreasing the removal percentage and capture capacity. The capture capacity at pH ~ 4 is less than half of that at pH ~ 7.4 (Fig. 2b). The effect of monovalent ion, Na+, on the (c) removal percentage and (d) capture capacity of ENCC, showing no significant impact because the equilibrium of carboxylate groups is not affected by monovalent ions. Effect of divalent ion, Ca2+, on the (e) removal percentage and (f) capture capacity of ENCC. The presence of 0.9 and 90 mM of Ca2+ decreased the DOX capture capacity of ENCC by a factor of 35% and 600%, respectively. This proves that the main drug capture mechanism is DOX-ENCC electrostatic attraction, disturbed by the divalent ion-mediated bridging of ENCC. In panels b, d, and f, the curve shows the Langmuir fit.