FIG 3.
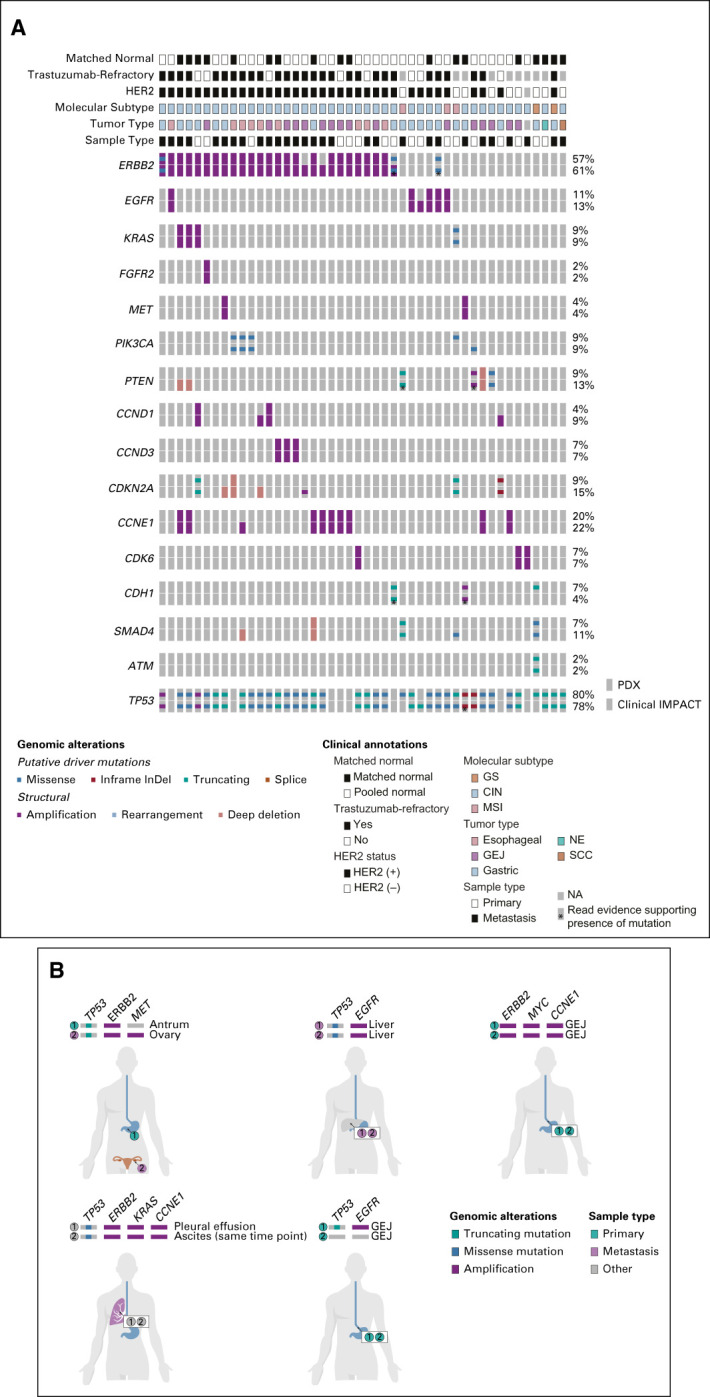
Genomic characterization of esophagogastric PDXs. (A) Relevant clinical features (tumor type, sample type of metastasis v primary tumor, patient HER2 status, and prior trastuzumab treatment) and key genomic alterations (mutations, structural alteration, and The Cancer Genome Atlas molecular subtype) in sequenced PDX and patient tumor samples. The percent alteration for each gene for PDX (top) and patient tumors (bottom) is provided. PDX sequencing was analyzed using either matched normal or a pooled normal as noted. Asterisk (*) denotes mutations present in clinical samples at low read counts below normal thresholds for mutation calling. (B) Representative examples of genomic alterations observed in two PDXs generated from the same patient. The site of origin (primary tumor or metastasis) is indicated. PDXs were generated at different time points unless indicated. CIN, chromosomal-instable; GEJ, gastroesophageal junction; GS, genomically stable; HER2, human epidermal growth factor receptor 2; MSI, microsatellite-instable; NA, not available; NE, neuroendocrine; PDX, patient-derived xenograft; SCC, squamous cell carcinoma; VUS, variants of unknown significance.
