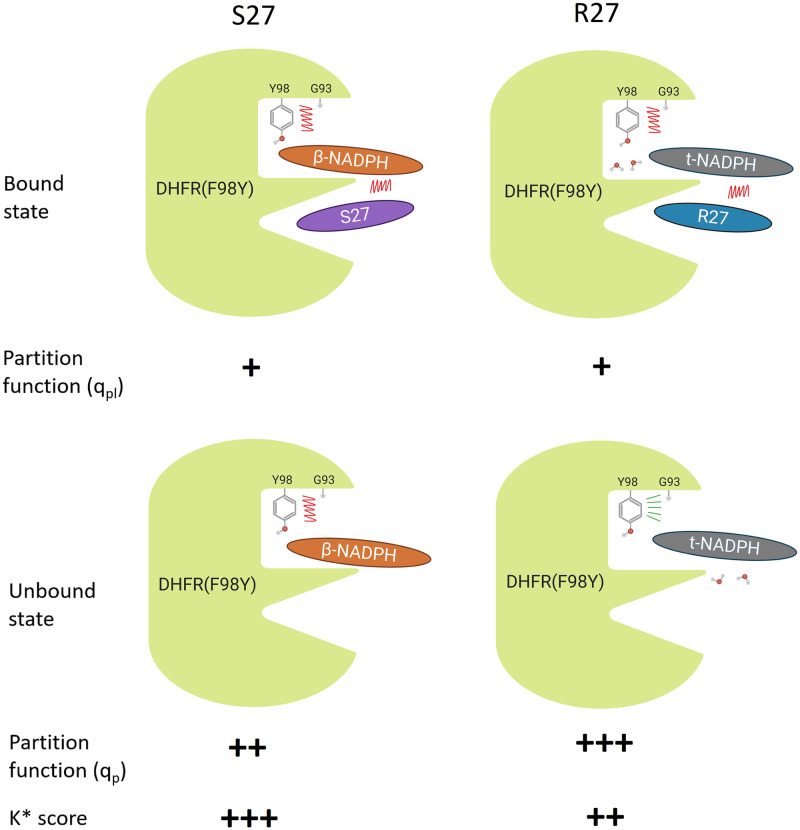
Fig 8. Proposed mechanism for chiral evasion of SaDHFR and resistance resilience by PLAs.
R-27 and S-27 have almost equal potency when bound to WT DHFR. However, S-27 binds significantly more tightly to F98Y DHFR than R-27 does. The different configuration of NADPH cofactor played an important role, which we term chiral evasion, according to our data. F98Y mutation introduces steric clashes (red jagged lines), and thus makes the whole complex energetically less favorable. By exploiting chiral inhibitor design, an inhibitor (S-27) from a PLA enantiomer pair was obtained that is resilient to chiral evasion. The opposite anomer R-27 is significantly less resilient. When R-27 binds to F98Y DHFR, it binds together with t-NADPH and also two water molecules that bridge the interaction. According to the osprey predictions, in the unbound state, the two water molecules move away from the binding pocket and move towards the space left by the absence of the inhibitor. Consequently, the crowding in the binding pocket is alleviated and the clashes can be relieved (green radial lines) in the unbound state. In contrast, when S-27 binds to F98Y DHFR, it only binds together with β-NADPH (without any water molecules) and the clashes cannot be relieved in the unbound state. Therefore, as a quotient of bound and unbound state partition function, the K* score (which approximates Ka) of S-27 is higher than that of R-27. This suggests S-27 should bind tighter to F98Y DHFR than R-27 does; furthermore this prediction agrees with IC50 data. Design for resilience to chiral evasion can therefore overcome drug resistance in a protein target.