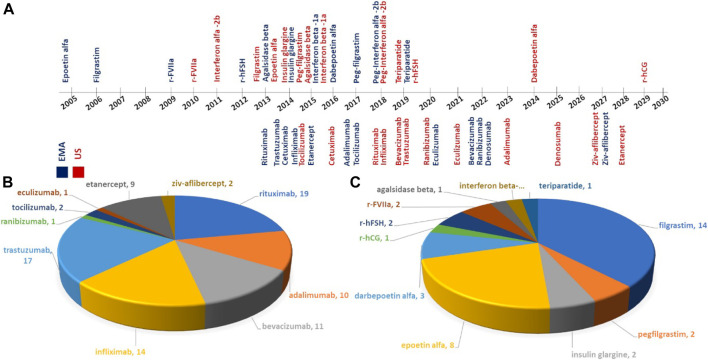
FIGURE 3.
(A) Timeline on patent expirations of reference products in European Medicines Agency; EMA (Moorkens et al., 2020) and U.S. Food and Drug Administration; USFDA (Derbyshire and Shina, 2019), publications on analytical similarity studies for biosimilars under (B) mAbs, and (C) non-mAbs.