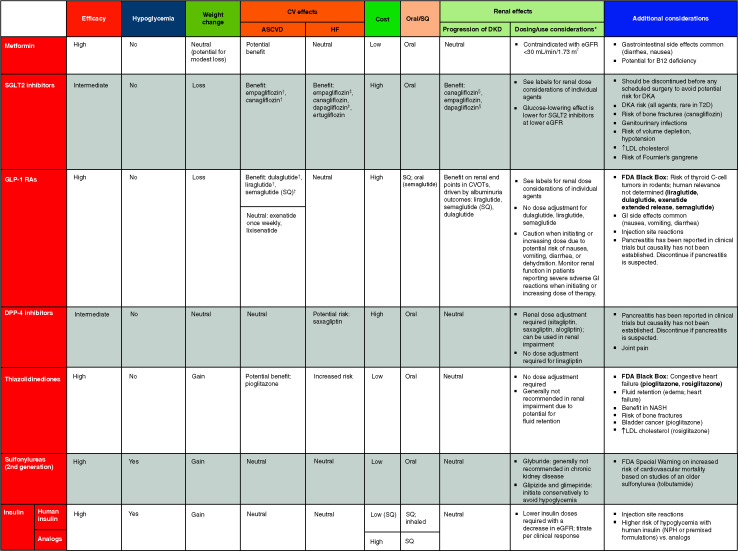
TABLE 9.2.
Drug-Specific and Patient Factors to Consider When Selecting Antihyperglycemic Treatment in Adults With Type 2 Diabetes
For agent-specific dosing recommendations, please refer to the manufacturers’ prescribing information.
FDA-approved for CVD benefit.
FDA-approved for HF indication.
FDA-approved for CKD indication. CVOT, cardiovascular outcomes trial; DPP-4, dipeptidyl peptidase 4; GLP-1 RA, glucagon-like peptide 1 receptor agonist; NASH, nonalcoholic steatohepatitis; SQ, subcutaneous; T2D, type 2 diabetes.