Abstract
Patient: Male, 37-year-old
Final Diagnosis: Immune-mediated myocarditis • pulmonary vasculitis • myositis • thrombocytopenia
Symptoms: Episode of hemoptysis with blood less than 100 ml • one-day history of sudden onset severe shortness of breath associated with orthopnea, dry cough and excessive sweating • reducing sensation to fine touch and temperature at the entire left upper limb • three-days history of back pain
Medication: —
Clinical Procedure: —
Specialty: General and Internal Medicine
Objective:
Unusual clinical course
Background:
The COVID-19 pandemic is a current global crisis, and there are hundreds of millions of individuals being vaccinated worldwide. At present, there have been few reports of COVID-19 vaccine-induced autoimmune processes manifested as myositis, thrombocytopenia, and myocarditis.
Case Report:
A 37-year-old man presented to the Emergency Department (ED) with a 3-day history of back pain and a 1-day history of left upper limb swelling with paresthesia and shortness of breath, 12-days after receiving the first dose of Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine. He was diagnosed with severe myositis complicated with rhabdomyolysis and non-oliguric acute kidney injury, thrombocytopenia, myocarditis with pulmonary edema, and pulmonary hemorrhage. Screens for potential toxic, infectious, paraneoplastic, and autoimmune disorders were unremarkable. The patient was treated with a 5-day course of intravenous methylprednisolone and intravenous immunoglobulin, with a good response. He was hospitalized for 16 days and discharged home on a tapering dose of oral prednisolone for 6 weeks.
Conclusions:
The case describes a possible link between Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine and immune-mediated myocarditis, pulmonary vasculitis, myositis, and thrombocytopenia. However, further data are required to confirm such an association.
Keywords: COVID-19 Vaccine, Hemorrhage, Myocarditis, Myositis, Thrombocytopenia
Background
There has been accelerated global efforts to develop and deliver effective vaccines to overcome the unprecedented pandemic caused by COVID-19. At the end of 2020, the U.S. Food and Drug Administration approved 2 mRNA vaccines for use through Emergency Use Authorization, namely the Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2) vaccines. To date, hundreds of millions of people have been vaccinated against SARS-CoV-2 infection using the BNT162b2 mRNA COVID-19 (Pfizer-BioNTech) vaccine worldwide. The BNT162b2 mRNA COVID-19 vaccine trial showed a high safety profile, with only a few major adverse drug reactions (ADRs), including axillary lymphadenopathy, ventricular arrhythmia, shoulder injury, and leg injury paresthesia [1]. Later, during the vaccination campaigns, allergic reactions were detected in 0.63% of the vaccinated people [2].
Additionally, other major ADRs were potentially linked to the BNT162b2 mRNA COVID-19 vaccine, including central nervous system thrombosis, deep vein thrombosis, and pulmonary embolism [3–5]. Also, thrombocytopenia and thrombosis have been mostly reported after AstraZeneca (ChAdOx1 nCoV-19) and Johnson & Johnson/Janssen COVID-19 vaccines [3]. However, several other rare ADRs, including 226 confirmed cases of myocarditis and others of pericarditis, occurred almost exclusively in young men after the second dose of vaccine and were linked to the mRNA COVID-19 vaccine [6–10]. We report a case of severe myositis, rhabdomyolysis, myocarditis, pulmonary hemorrhage, and thrombocytopenia in a previously healthy 37-year-old man 12 days following the first dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine.
Case Report
A 37-year-old man presented to the Emergency Department (ED) at the Sultan Qaboos University Hospital in July 2021 with a 3-day history of back pain and 1-day history of left arm pain, swelling, and paresthesia. Also, he reported a 1-day history of sudden-onset severe shortness of breath associated with orthopnea, dry cough, and excessive sweating. There was no history of chest pain, palpitations, dizziness, syncope, fever, weakness, bowel or urinary incontinence, arthritis, oral or perianal ulcers, rash, or alopecia. The patient denied any history of unaccustomed exercise, body trauma, or prolonged exposure to the sun. The patient never smoked cigarettes and denied active alcohol consumption and drug abuse. The patient had no medical background, did not take medications before this illness, and did not have a food or medication allergy. He did not have a previous COVID-19 infection and he received the first dose of the Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine 12 days before the onset of these symptoms. There was no family history of sudden cardiac death, autoimmune diseases, or musculoskeletal diseases.
Upon presentation to the ED, he was alert and oriented but looked dehydrated. His vital signs were as follows: temperature 37.1°C, blood pressure 138/87 mm Hg, heart rate 114 beats/min, respiratory rate 32 breaths/min, and oxygen saturation of 78% on ambient air, which then increased to 95% with 8 L of oxygen.
Chest auscultation revealed widespread bilateral fine crepitation, mainly over the left lung. His left upper arm at the vaccination site was markedly swollen, stiff, warm, and tender, with mild skin erythema and restricted movement at the left shoulder and elbow joints. The left upper arm circumference was 40 cm, and the right upper arm circumference was 33 cm. The peripheral pulse was felt with good volume, capillary refill time was <2 s, and no xerostomia was noted.
The patient also reported a reduction in sensation to fine touch and temperature at the entire left upper limb. The spine examination revealed tenderness at paraspinal muscles at the L4 to S1 vertebral level, with severe tenderness over the latissimus dorsi muscle. The straight leg test was negative. The neurovascular examinations of both lower limbs and the right upper limb were intact. Other systemic examinations were un-remarkable. The patient had tea-colored urine, and a urine dip-stick examination was positive for blood (+4) and protein (+).
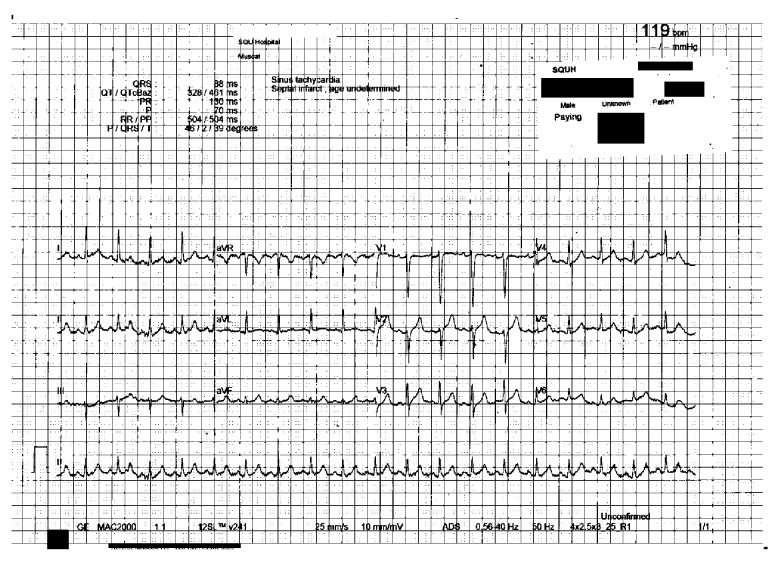
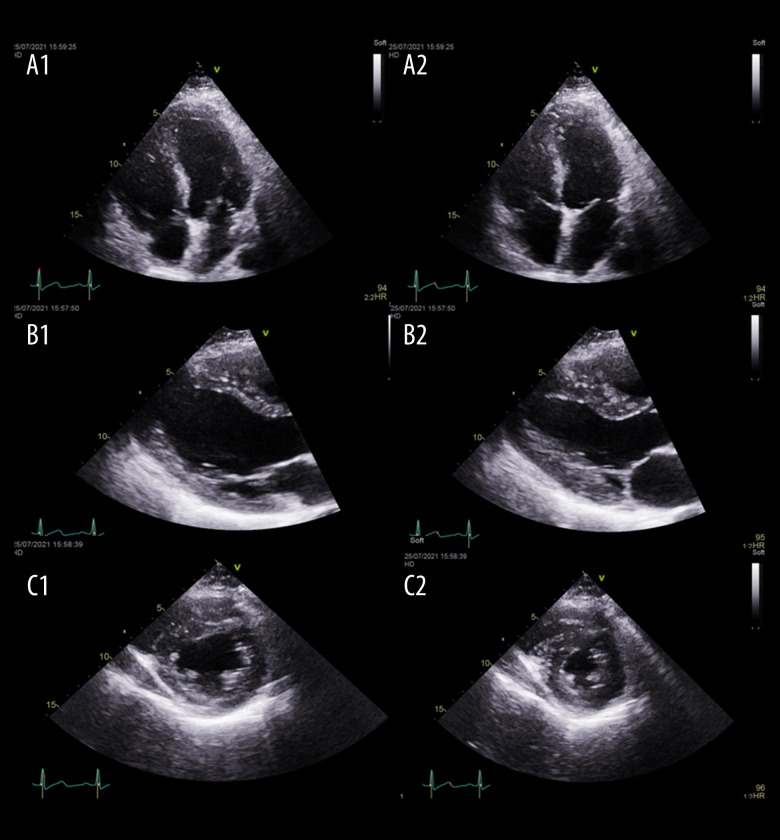
The laboratory findings upon admission are presented in Table 1. As summarized, the patient had neutrophilic leukocytosis, raised D-dimer level, high creatinine kinase (CK) level, acute kidney injury, hyperkalemia, and hypoxemia, with partially compensated high anion gap metabolic acidosis. An initial 12-lead electrocardiography (ECG) showed sinus tachycardia with no significant ST-segment or T-wave changes (Figure 1), and he had raised troponin and ProBNP. An Xpert Xpress PCR test of SARS-CoV-2 obtained from a nasopharyngeal swab was negative. His blood culture, urine culture, and virology screens were unremarkable (Table 2). The chest X-ray showed features suggestive of pulmonary edema (Figure 2). A bedside chest ultrasound showed bilateral B lines. A computed tomography scan (CT) of the chest and pulmonary angiogram showed extensive bilateral ground-glass and patchy nodular opacities on the left lung; there was no pulmonary embolism (Figure 3). An echocardiogram reported mild concentric left ventricular hypertrophy with mild global hypokinesis of the left ventricle, and the overall left ventricular systolic function was mildly impaired, with an ejection fraction between 45% and 50%. The right ventricular systolic function was mildly impaired, and there was no pericardial effusion (Figure 4). A left upper arm soft tissue ultrasound showed soft tissue edema and abnormal heterogeneous echogenicity and relatively increased vascularity, which are features suggestive of myositis-myonecrosis. Left shoulder magnetic resonance imaging showed diffuse subcutaneous edema and abnormal signal intensity of the deltoid muscle, infraspinatus, and supraspinatus muscles, likely representing myositis changes (Figure 5). However, clinically and radiologically, there were no features suggestive of necrotizing fasciitis or compartment syndrome.
Table 1.
Results summary of the presenting laboratory tests.
| Test | Result | Normal range |
|---|---|---|
| Hematology labs | ||
| Hb (g/L) | 18.1 | 11.5–15.5 |
| Haematocrit (L/L) | 0.579 | 0.350–0.450 |
| Platelet count (109/L) | 245 | 150–450 |
| White cell count (109/L) | 28.7 | 2.2–10.0 |
| Neutrophils (109/L) | 23.1 | 1.0–5.0 |
| D-dimer (mg/L FEU) | 5.0 | 0.2–0.7 |
| Biochemical labs | ||
| CRP (mg/L) | 11 | 0–5 |
| Troponin T (ng/L) | 1331 | <14 |
| CK (U/L) | 93046 | 39–308 |
| ProBNP (pg/ml) | 2811 | 20–85 |
| Cardiac isoenzyme of CK (U/L) | 1290.9 | 0.0–25.0 |
| eGFR (ml/min/1.73 m2) | 23 | >90 |
| Venous pH | 7.19 | 7.35–7.45 |
| PCO2 (mmHg) | 45.3 | 36–48 |
| HCO3 (mmol/L) | 15 | 21.8–26.9 |
| Lactate (mmol/L) | 5.6 | 0.5–1.6 |
| Anion gap | 23 | 5–13 |
| Electrolytes | ||
| Potassium (mmol/L) | 5.6 | 3.5–5.1 |
| Sodium (mmol/L) | 134 | 135–145 |
| Calcium, albumin adjusted (mmol/L) | 1.84 | 2.15–2.55 |
| Phosphate (mmol/L) | 2 | 0.81–1.45 |
Hb – hemoglobin; CRP – C reactive protein; CK – creatine kinase; ProBNP – N-terminal pro B-type natriuretic peptide; eGFR – estimated glomerular filtration rate.
Figure 1.
Twelve-lead electrocardiography obtained on the day of presentation.
Table 2.
Results summary of the hospitalization microbiology tests.
| Test | Result |
|---|---|
| Respiratory viral screen (including: Rhinovirus PCR, RSV PCR, Adenovirus RVS, Enterovirus RVS, H1N1 confirmatory PCR, Human Metapneumovirus PCR, Parechoviruses, Human Bocavirus, Influenza A and B PCR, Corona viruses 229, 63 and 43 HKU PCR, Parainfluenzae 1,2,3,4 PCR) | Negatives |
| Mycoplasma pneumonia PCR | Negative |
| Urinary antigen (Legionella) | Negative |
| Urinary antigen (pneumococcus) | Negative |
| Pneumocystis PCR | Negative |
| Cytomegalovirus serology (CMV) | Negative |
| Epstein Barr Virous Serology (EBV) | Negative |
| Toxoplasma Serology | Negative |
| HIV 1&2 Ag/Ab | Negative |
| Urine culture | Negative |
| Blood culture | Negative |
| Sputum culture | Negative |
| Mycobacterium Tuberculosis (TB) gene Expert | Negative |
| Aspergillus Galactomannan Antigen | Negative |
Figure 2.
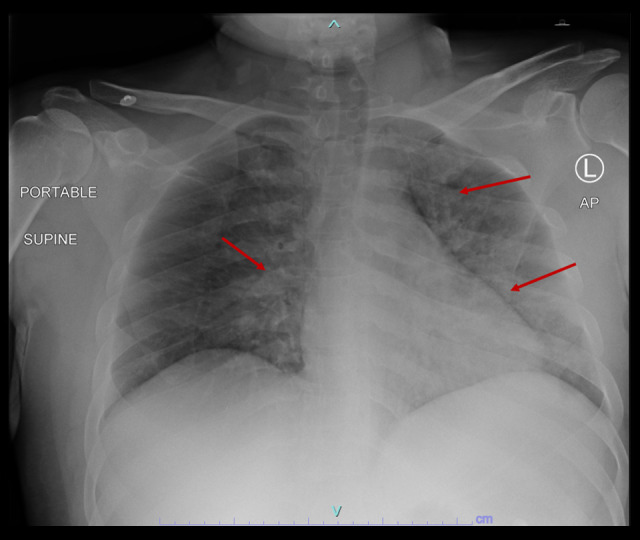
Chest X-ray on day of presentation showing extensive bilateral pulmonary infiltrates (red arrows).
Figure 3.
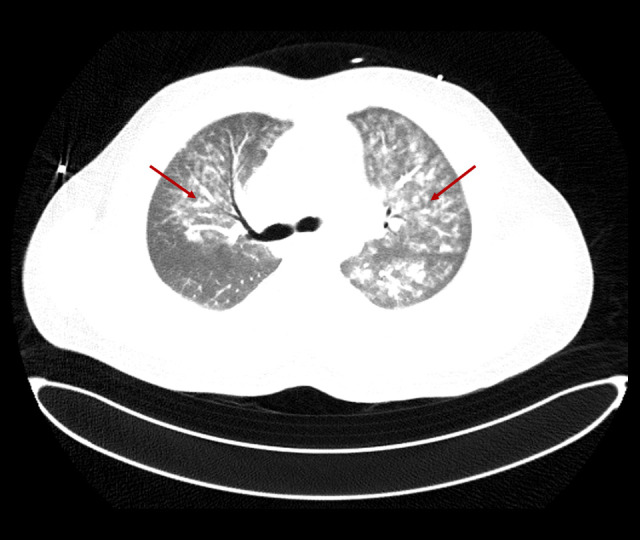
Chest computed tomography with contrast showing extensive bilateral ground-glass and patchy nodular opacities, more on the left lung (red arrows).
Figure 4.
Echocardiogram at presentation demonstrating the following views: (A1) 4-chamber view in diastole; (A2) 4-chamber view in systole; (B1) parasternal long-axis view in diastole; (B2) parasternal long-axis view in systole; (C1) parasternal short axis view in diastole; and (C2) parasternal short axis view in systole.
Figure 5.
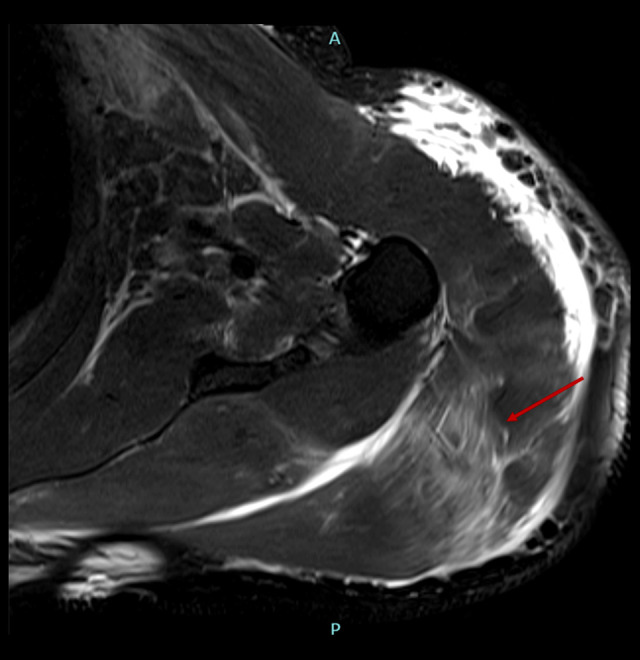
Magnetic resonance imaging T2 fat-suppressed axial sequence of the left shoulder with diffuse subcutaneous edema and abnormal signal intensity of deltoid muscle, infraspinatus, and supraspinatus muscles, likely representing myositis changes (red arrow).
The initial working diagnosis was myositis, myocarditis with pulmonary edema, and rhabdomyolysis complicated with severe pre-renal and non-oliguric acute kidney injury. The patient was managed with aggressive hydration using intravenous (i.v.) normal saline and sodium bicarbonate for urine alkalization. Also, he was started on i.v. furosemide, targeting urine output of more than 100 mL per h. Owing to the pulmonary edema, he was kept on continuous positive airway pressure. Other management included empiric antimicrobial coverage for pneumonia (2 g of ceftriaxone daily and 100 mg of doxycycline 2 times daily), pain management (1 g paracetamol every 6 h, i.v. tramadol 50 mg 4 times daily as needed), and physiotherapy.
On the second day of hospitalization, a full blood count showed thrombocytopenia. Given the close interval between the COVID-19 vaccine and the onset of his symptoms, although there was no evidence of thrombosis, a blood sample was sent for a heparin-induced thrombocytopenia (HIT) rapid screen to check for evidence suggesting vaccine-induced immune thrombotic thrombocytopenia; however, the HIT screen came back negative. Also, vasculitis screens, including ANA, anti-GBM, and antiphospholipid were all negative, apart from a weakly positive cANCA. However, PR3 and MPO were both negative. Given an unremarkable workup and owing to the proximity of the COVID-19 vaccine and patient’s symptoms, we suspected a generalized vaccine immune-mediated process causing myocarditis, and myositis; therefore, the patient was started on i.v. human normal immunoglobulin (IVIG) (1 g/kg/day) and i.v. methylprednisolone (1000 mg/day). He started to have an episode of hemoptysis on the same day, with blood less than 100 mL, and his hemodynamic and respiratory statuses were unchanged. It was observed that there was a steady decline in hemoglobin level since the admission. A bronchoscopy examination was done and showed evidence of pulmonary alveolar hemorrhage, which might indicate pulmonary vasculitis.
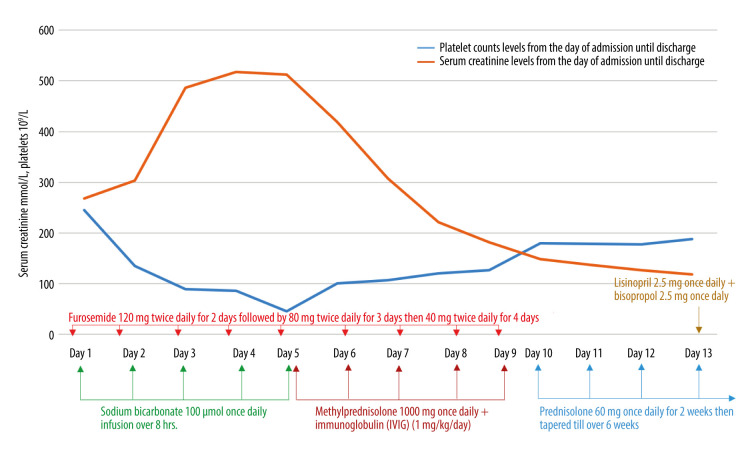
Following the initiation of IVIG and methylprednisolone, the patient showed a remarkable improvement, evidenced by a reduced oxygen requirement; the troponin (Figure 6), CK (Figure 7), serum creatinine levels and platelet count were also improved (Figure 8). He was weaned from oxygen on day 7 of hospitalization. He received methylprednisolone and IVIG injections for 5 days, followed by oral prednisolone 60 mg once daily for 2 weeks, followed by a tapering regimen for a total of 6 weeks till off, as well as tablets lisinopril 2.5 mg once daily and bisoprolol 2.5 mg once daily. The patient was discharged after 16 days of hospitalization. Post-discharge follow-up revealed complete resolution of the patient symptoms. Thus, the case was reported to the Vaccine Adverse Events Reporting System.
Figure 6.
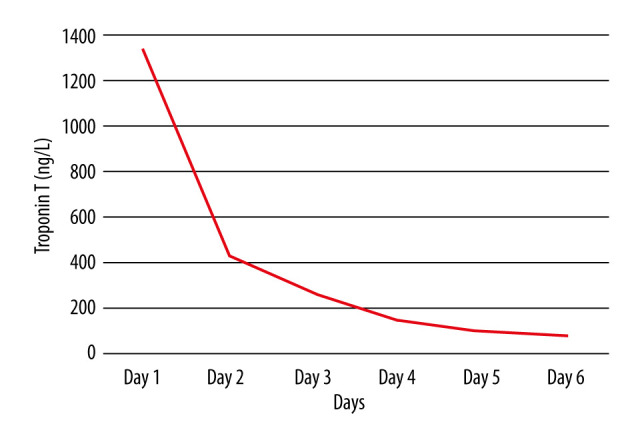
Trend of troponin T (ng/L) during hospitalization.
Figure 7.
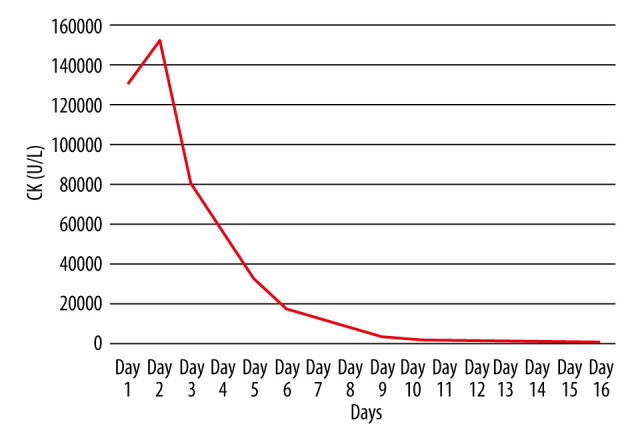
Trend of creatine kinase levels (U/L) during hospitalization.
Figure 8.
Trend of serum creatinine levels (µmol/L) and platelet counts (109/L) during hospitalization.
Discussion
The case describes a possible link between the mRNA COVID-19 vaccine and immune-mediated myocarditis, pulmonary vasculitis, myositis, and thrombocytopenia in a young, healthy man who presented to the hospital 12 days after vaccination.
Our patient had an extensive myositis complicated with rhabdomyolysis, which could have been due to muscle injury at the injection site that led to the host’s inflammatory response against the intramuscular vaccination, causing severe muscle toxicity and thus releasing high CK levels [11]. Shoulder injuries were reported in the main BNT162b2 mRNA COVID-19 vaccine trial [1]. Our patient was kept initially without prophylaxis anticoagulation owing to suspicion of vaccine-induced immune thrombotic thrombocytopenia, which was ruled out when the HIT screen was negative a few days later. By this time, the ceftriaxone was also discontinued to prevent a further drop in platelet count, given the possible drug-induced thrombocytopenia [12]. On day 4 of admission, the patient started to have hemoptysis of less than 100 mL, for which he underwent bronchoscopy, showing pulmonary hemorrhage. All relevant auto-immune workup results were negative (Table 3).
Table 3.
Results summary of the hospitalization autoimmune tests.
| Test | Result |
|---|---|
| Anti-nuclear antibody (ANA) | Negatives |
| Anti-Glomerular Basement Membrane Disease (Goodpasture’s Syndrome) | Negative |
| Autoimmune antiphospholipid syndrome | Negative |
| Anti-neutrophils cytoplasmic antibody | Negative |
Over the last decades, several clinical trials and observational studies have shown the link between some vaccines and vasculitis resulting in pulmonary hemorrhage and severe thrombocytopenia [13]. Also, a recent case report presented the occurrence of leukocytoclastic vasculitis after the mRNA COVID-19 vaccine [14]. Our patient might have had pulmonary vasculitis resulting in pulmonary hemorrhage, which responded well to IVIG and systematic steroids.
The patient also had myocarditis, which was evident by pulmonary edema, raised troponin and pro-BNP levels, and reduced left ventricular systolic function on echocardiogram. Acute coronary syndrome was ruled out clinically owing to absent chest pain and ischemic or dynamic ECG changes in follow-up ECGs. The patient denied any recent upper respiratory tract infection. His virology screen and autoimmune workup were also negative. Hence, vaccine-induced myocarditis was more likely, especially in view of the reported cases of myocarditis linked to the mRNA COVID-19 vaccine [6–10]. The exact mechanism of COVID-19 vaccine-induced myocarditis is still not clear. However, there are a few proposed hypotheses, including anti-idiotype cross-reactive antibody-mediated cytokine expression in the myocardium and induction of apoptosis and non-specific innate inflammatory responses between viral spike protein and unknown cardiac protein [15].
Our patient was treated initially with aggressive hydration with high-volume i.v. normal saline for rhabdomyolysis, as he was clinically severely dehydrated, along with i.v. furosemide for pulmonary edema. To date, there is no randomized controlled trial that provides the best treatment modalities of acute myocarditis; most of the evidence available suggests direct treatment toward the heart failure and arrhythmia caused by myocarditis [16].
After excluding infectious, toxic, and autoimmune processes, the patient was kept on methylprednisolone (1000 mg/day for 5 days) and IVIG (1g/kg/day for 5 days), followed by oral prednisolone 60 mg once daily. The patient showed remarkable improvement clinically, biochemically, and hemodynamically; therefore, a decision was made to continue predniso-lone oral tapering doses for 6 weeks.
This case describes a possible link between BNT162b2 mRNA COVID-19 (Pfizer-BioNTech) vaccine and ADRs manifested by thrombocytopenia, alveolar hemorrhage, myocarditis, and myositis complicated with rhabdomyolysis and non-oliguric acute kidney injury after we excluded all other causes of the patient presentation. In view of the proposed immune-mediated effect of the presented complications, the patient was managed with IVIG and steroids, which resulted in significant improvement in his clinical condition through the course of admission.
Conclusions
This case demonstrates a possible rare complication of myositis, thrombocytopenia, myocarditis, and pulmonary vasculitis hemorrhage in a 37-year-old man 12 days after receiving the Pfizer-BioNTech vaccine. Currently, there are a few reports of myositis, myocarditis, and thrombocytopenia among COVID-19 vaccine recipients, which are more likely to be immune-mediated in etiology. However, further data and confirmatory testing such as histopathological examination are required to support such an association.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji A, Wickner PG, Saff R, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–37. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):e92. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carli G, Nichele I, Ruggeri M, et al. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16(3):803–4. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Maqbali JS, Al Rasbi S, Kashoub MS, et al. A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 days following a first dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Am J Case Rep. 2021;22:e932946. doi: 10.12659/AJCR.932946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Disease Control and Prevention . Myocarditis pericarditis following mRNA COVID-19 vaccination. Centers for Disease Control and Prevention; US: 2021. [Google Scholar]
- 7.D’Angelo T, Cattafi A, Carerj ML, et al. Myocarditis after SARS-CoV-2 vaccination: A vaccine-induced reaction? Can J Cardiol. 2021;37(10):1665–67. doi: 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei R, Raschi E, Forcesi E, et al. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183–86. doi: 10.1016/j.ijcard.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6(10):1202–6. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassar M, Nso N, Gonzalez C, et al. COVID-19 vaccine-induced myocarditis: Case report with literature review. Diabetes Metab Syndr. 2021;15(5):102205. doi: 10.1016/j.dsx.2021.102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodorou DJ, Theodorou SJ, Axiotis A, et al. COVID-19 vaccine-related myositis. QJM. 2021;114(6):424–25. doi: 10.1093/qjmed/hcab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold DM, Kukaswadia S, Nazi I, et al. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost. 2013;11(1):169–76. doi: 10.1111/jth.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonetto C, Trotta F, Felicetti P, et al. Vasculitis as an adverse event following immunization – systematic literature review. Vaccine. 2016;34(51):6641–51. doi: 10.1016/j.vaccine.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Bostan E, Gulseren D, Gokoz O. New-onset leukocytoclastic vasculitis after COVID-19 vaccine. Int J Dermatol. 2021;114(6):424–25. doi: 10.1111/ijd.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and pericarditis following mRNA COVID-19 vaccination: What do we know so far? Children (Basel) 2021;8(7):607. doi: 10.3390/children8070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurwitz B, Issa O. Management and treatment of myocarditis in athletes. Curr Treat Options Cardiovasc Med. 2020;22(12):65. doi: 10.1007/s11936-020-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]