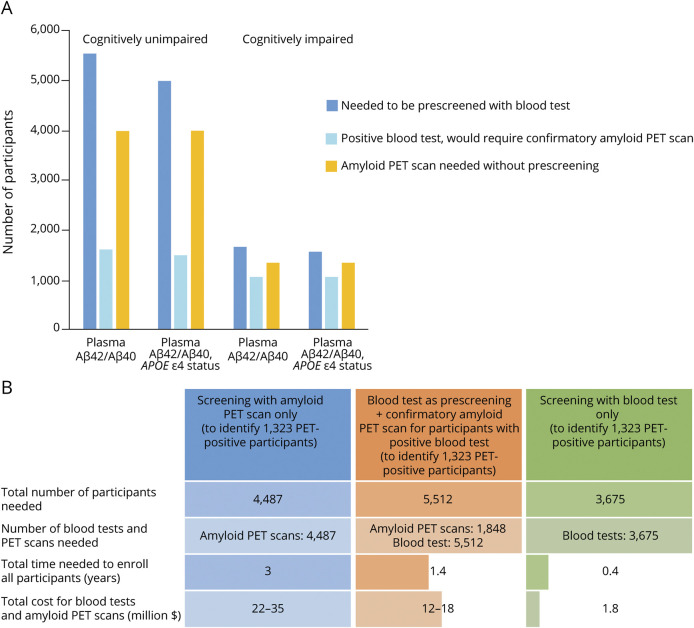
Figure 3. Plasma Aβ42/Aβ40 as a Prescreening Test for Brain Amyloidosis.
(A) The number of participants and amyloid PET scans needed to identify 1,000 amyloid PET–positive participants, with and without prescreening with a blood test. The amyloid PET positive rate was assumed to be 25% for the cognitively unimpaired group and 75% for the cognitively impaired group. Data provided in eTable 6, links.lww.com/WNL/B715, were used as the sensitivity and specificity of plasma Aβ42/40 test. (B) The time and cost savings associated with prescreening for brain amyloidosis with plasma Aβ42/Aβ40 were evaluated for an Alzheimer disease prevention trial similar to the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) Study. In the A4 study, amyloid PET scans were performed in 4,487 participants over a 3-year period, and 1,323 participants (30%) were classified as amyloid PET positive. The sensitivity of 0.80 and specificity of 0.83 based on cognitively unimpaired participants as shown in eTable 6, links.lww.com/WNL/B715, were used for the accuracy of the blood test. Costs were assumed to be $5,000–$8,000 per amyloid PET scan and $500 per blood test.