Figure 1. Constitutive HER2-HER3 signaling does not require canonical ECD-driven dimerization-inducing events.
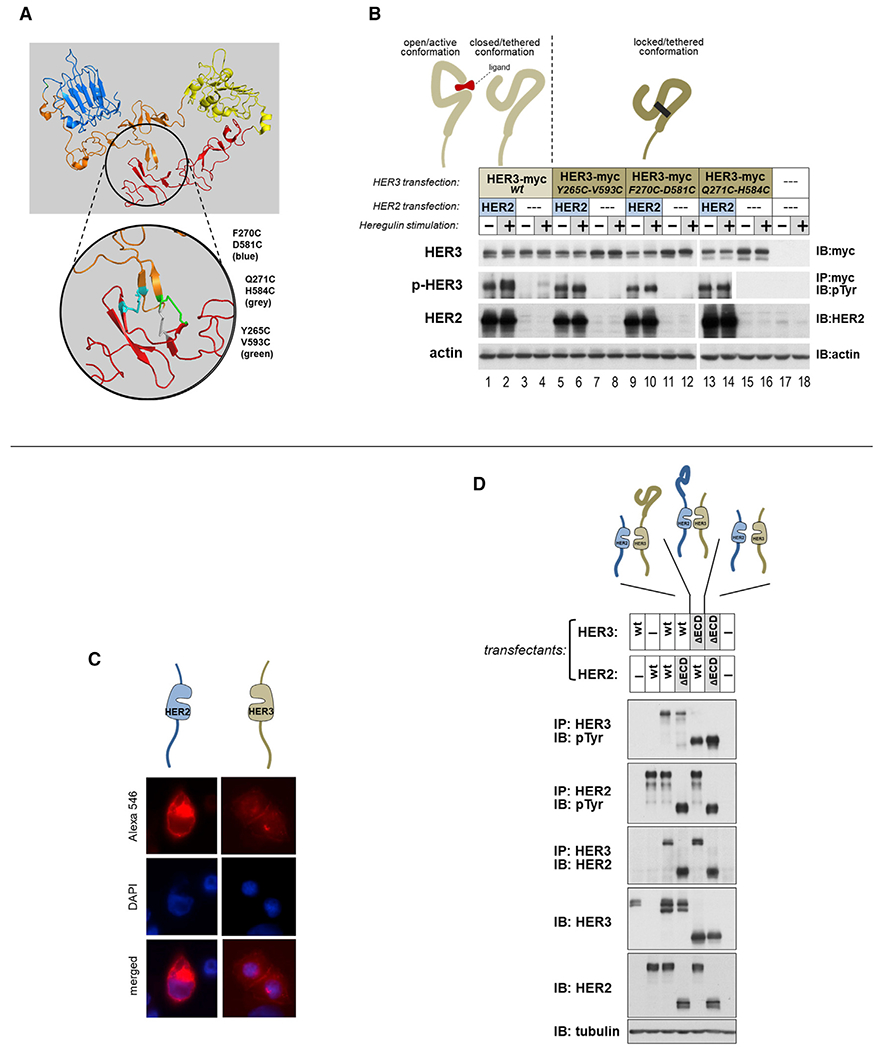
(A) The ECD of HER3 was locked in the closed/tethered conformation by introducing double cysteine mutations at the indicated nearby residues of domains II and IV. Three different locked versions of HER3 were generated by mutating the indicated pairs of residues to cysteines as indicated. Of these, the Y265C/V593C double mutant has been studied extensively before and confirmed to be locked in the closed conformation by disulfide bridging (Kani et al., 2005).
(B)CHO-K1 cells were transfected to express HER2 and HER3 mutants as indicated. Wild-type HER3 is constitutively phosphorylated in the presence of overexpressed HER2 (lane 1)and is further inducible by ligand stimulation (lane 2). There is also slight induction of HER3 phosphorylation by the background low level of endogenous HER2 in CHO-K1 cells (lane 4). The locked HER3 mutants, in the presence of overexpressed HER2, are fully competent at constitutive phosphorylation despite the fact that they are not able to adopt the open conformation and expose their dimerization interface and have lost ligand responsiveness (lanes 5–16).
(C) The entire ECDs of HER2 and HER3 were deleted, preserving the N-terminal signal sequences. Membrane localization of these constructs was confirmed by immunofluorescence staining as indicated.
(D) These constructs are fully competent at constitutive phosphorylation when HER2 is overexpressed, despite complete loss of ECD functions.
